G945
Fertilizers for Vegetables in Home Gardens
This NebGuide discusses how to manage soil and nutrients when growing vegetables, including soil testing, soil pH, organic matter and the use of commercial fertilizers.
David D. Tarkalson, Extension Soils Specialist
Dale T. Lindgren, Extension Horticulturist
Charles A. Shapiro, Extension Soils Specialist
Laurie Hodges, Extension Commercial Vegetable Specialist
- Soil Sampling and Testing
- Soil pH
- Raising Soil pH
- Lowering Soil pH
- Manufactured (Commercial) Fertilizers
- Nitrogen
- Phosphorus
- Other Nutrients
- Adjusting Fertilizer Based on Your Garden Area
- Applying Materials When a Weight Scale is Not Available
- Acknowledgment
|
Growing vegetables in home gardens is a hobby for some, a source of nutritious food for others and an income source for other backyard farmers. Research has demonstrated that proper fertility can increase vitamins, minerals and antioxidants in vegetables. Correct fertility is also vital to optimize production quantity and quality of vegetables.
Soil Sampling and Testing
Fertilizer is used to provide nutrients to plants not adequately supplied by the soil. A soil test indicates which nutrients to add and whether soil amendments such as lime or organic matter are needed. Soils do not need to be tested every year. A test every fifth year is adequate unless a major soil amendment was added since the last test.
Unless there are obvious soil differences in the garden, one bulk soil sample consisting of 10-15, eight-inch deep cores randomly collected in the garden area is recommended. A “Soil Sample Information Sheet” and soil containers are available at extension offices or online at http://agronomy.unl.edu/spal/. The form should be submitted with the soil sample. On the back of the form are some general guidelines for taking soil samples. The most common and useful soil tests include pH, buffer pH, organic matter, phosphorus and potassium.
Soil pH
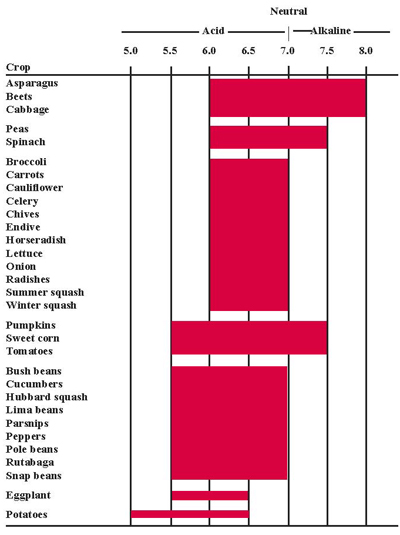
The pH of a soil is a measure of acidity or alkalinity. The numbers reported from a soil test generally range from 4.5 to 8.5. A pH of 7.0 is neutral. Those below 7.0 are acidic and those above 7.0 are alkaline. Figure 1 shows the best pH ranges for various vegetable crops. A pH of 6.5 is ideal for most vegetables. Below pH 5.5 or above 7.5, soil modification often is necessary. Soil pH directly influences the availability of many nutrients. Maintaining a proper pH is important to avoid nutrient deficiencies and toxicities in vegetables.
Raising Soil pH
Lime materials can raise soil pH if applied at the correct rate and using the correct application method. Apply lime to soils with a pH below 5.5. The rate required to raise the soil pH to 6.5 to a depth of 6 to 8 inches is determined by the buffer pH test and the neutralizing value of the lime material. The neutralizing value [effective calcium carbonate equivalent (ECCE)] of liming materials is listed on the product label. The soil test shows the amount of lime to apply per 1,000 square feet based on the buffer pH of the soil and a lime material with a neutralizing value of 60 percent or 100 percent (neutralizing value used depends on the laboratory where the sample is sent). Many lime materials have neutralizing values different than 60 or 100 percent. Different kinds of lime can be used. Common lime materials available at garden stores include crushed limestone, dolomite, burned lime, quick lime, hydrated lime and slake lime. To determine the pounds (lbs) of the lime material to apply to your garden use Table I. For best results, incorporate the lime into the soil with a rototiller to 6 inches. Lime reacts slowly in soil so it may take one to three years for the pH to peak at its highest level.
|
|||||||||||||||||||||||||||||||||||
Do not over-lime. Apply lime according to the results of a soil test. Excess lime is likely to result in iron chlorosis in plants. If lime can’t be incorporated to 6 inches, apply proportionately less now and the remainder after a few years.
Lowering Soil pH
If soil pH is higher than 7.5, which is common in western Nebraska, it may be desirable to lower the soil pH. This generally is not practical for large areas due to the cost, but it may be feasible on small garden plots or when a high value crop requires a low soil pH. Elemental sulfur can be used to lower soil pH.
Elemental sulfur is available for purchase and is most commonly used. Table II shows the amount of sulfur to add, depending on the texture and pH of the soil. The amount shown is the approximate quantity to apply. For best results, incorporate the elemental sulfur into the soil to a depth of 6 inches with a rototiller or rake in the fall.
Sulfur reacts slowly in the soil and may take a year or more to change the soil pH. A soil test should be done again one or two years after sulfur is applied to determine if more sulfur is needed. Irrigation water can be high in calcium carbonate and will neutralize the effect of the sulfur over time. Therefore, elemental sulfur may need to be applied every few years. To determine when to apply, periodically test soil pH.
|
|||||||||||||||||||
Manufactured (Commercial) Fertilizers
Many commercial fertilizers are available in different nutrient grades and analysis. Nebraska state law requires that the label on all fertilizers sold show a guaranteed amount of nitrogen (N), phosphorus (P2O5), and potassium (K2O). A nutrient analysis of 10-20-10 means it is guaranteed to contain 10 percent N, 20 percent P2O5, and 10 percent K2O.
The content of other nutrients, such as sulfur, iron or zinc, also must be listed if the fertilizer manufacturer wants to guarantee the amount.
The rate of fertilizer application depends on the nutrient analysis of the fertilizer and the amount of nutrient to be applied. To determine the pounds of fertilizer to apply to your garden, use Equation 1. A spreadsheet calculator for all the equations in this NebGuide is available for downloading at http://soilfertility.unl.edu/. Click on Software.
Equation 1
| Pounds fertilizer material/1,000 sq ft = |
lb nutrient needed /1,000 sq ft |
(Percent of nutrient in fertilizer material ÷ 100) |
If one pound of nitrogen per 1,000 sq ft is needed and a material with a nutrient analysis of 10-20-10 is used, it is necessary to apply 10 lb of 10-20-10 per 1,000 sq ft to get one pound of nitrogen. This 10-pound rate of 10-20-10 fertilizer also results in the application of 2 lb of P2O5 and 1 lb of K2O.
Common fertilizer materials containing nitrogen, phosphorus and potassium include ammonium sulfate (21-0-0), urea (46-0-0), triple super phosphate (0-46-0), and potassium chloride (muriate of potash) (0-0-60).
Most fertilizers purchased in small amounts from garden centers are a mixture of nitrogen, phosphorus and potassium. It is important to consider the fertility needs of the plants and the nutrients present in the soil and buy the grade of material that supplies the needed nutrients.
Both liquid and dry manufactured fertilizers can be equally effective in supplying nutrients to plants, provided soil moisture is sufficient for plants to take up the nutrients.
Most research has shown that garden amendments or fertilizers that contain additives such as bio-stimulants, vitamins and some micronutrients often do not increase plant production.
|
||||||
Nitrogen
Nitrogen is the nutrient that plants need from the soil in the largest quantity. When organic matter decomposes in the soil, nitrogen becomes available for plant use. However, this nitrogen is not adequate to meet the demands of many vegetable plants, so additional nitrogen from fertilizer or manure is needed. If manure is used in a vegetable garden, be sure to wash vegetables thoroughly, and peel and cook them to remove potential pathogens. For most vegetables, approximately 2 lb of available nitrogen per 1,000 sq ft is adequate for early plant growth.
Fertilizer application rates are divided into preplant (before planting) and sidedress (when plant is growing) timings. Table III lists suggested preplant nitrogen rates. Table III is based on research that shows that approximately 0.4 lb of nitrogen per 1,000 sq ft per year is released for each percent of organic matter in soil. Total nitrogen requirements and sidedress timing for various vegetables are shown in Table IV. Asparagus, beans and radishes have a nitrogen recommendation of less than 2 lb nitrogen per 1,000 sq ft and preplant nitrogen applications should be adjusted based on soil organic matter. Sidedress application rates are determined using Equation 2:
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Equation 2
| Sidedress nitrogen application rate (lb/1,000 sq ft)* = Total nitrogen needed (Table IV) – Preplant nitrogen application rate (Table III) – (% organic matter x 0.4) |
| *If the result is 0 or a negative number, do not apply sidedress nitrogen. |
If sufficient manure is applied, you may not need to apply preplant or sidedress nitrogen this year.
If using a slow release fertilizer, apply the total crop nitrogen requirement at preplant. Most slow release fertilizers will release nutrients over the entire growing season (check label for this information). Slow release fertilizers typically are conventional fertilizers with a coating that causes nutrients to be released over an extended time.
Nitrogen is a mobile nutrient in soil and can be applied when needed by the plants. Nitrogen can be applied to the soil surface and incorporated with water or tilled into the soil. High concentrations of nitrogen fertilizer should not be placed in contact with seeds or growing plants because injury is likely. Place fertilizer at least two inches away from the stem and scattered across the soil surface.
Be careful not to apply too much nitrogen. Nitrogen can promote excessive vegetative growth and delay vegetable fruit production. Nitrogen and phosphorus applied at rates exceeding the recommended rates can be harmful to the environment.
Incorporation of materials with high amounts of carbon in relation to nitrogen, such as wood-based mulches and tree leaves, can result in nitrogen deficiencies in vegetable crops. An additional 0.5 lb nitrogen per 1,000 sq ft should be applied under these circumstances. Incorporation of grass clippings and manures does not require additional nitrogen due to their higher nitrogen content.
Phosphorus
Phosphorus deficiencies in plants can develop in some Nebraska soils and are best identified with a soil test. Table V gives the suggested phosphorus rates based on a soil test. Besides commercial phosphorus fertilizers, animal manures are a good source of this nutrient.
Starter fertilizer — one high in phosphorus and applied in a band beside the row at planting — often stimulates early growth of certain crops or transplants (e.g., sweet corn or popcorn), but seldom increases yield on high phosphorus soils.
Phosphorus is a non-mobile nutrient in the soil and should be applied prior to or at planting. Phosphorus fertilizer can be broadcast on the soil and incorporated by tillage or it can be applied in a separate row beside the seed row at the time of planting. Row-applied fertilizer should not be placed in direct contact with the seed.
|
||||||||||||||||||||
Other Nutrients
Potassium is usually high in Nebraska soils, but occasionally a soil test may indicate a lower level. The suggested potassium rate, based on soil test, is given in Table VI. Potassium should be applied prior to tillage.
Sulfur deficiency can occur on very sandy, low organic matter soils, particularly in north central Nebraska. A few vegetables, such as the cole crops (cabbage, broccoli, etc.), require higher levels of sulfur than other vegetables. The application of organic materials, such as manure, will help correct the problem. Many fertilizers include small amounts of sulfur, which will be indicated on the fertilizer analysis found on the package label. These small amounts are often sufficient to overcome soil deficiencies. Using ammonium sulfate as a source of nitrogen fertilizer supplies more than adequate amounts of sulfur.
|
|||||||||||||||
Iron chlorosis may occur in some soils that contain excess lime and are compacted and poorly drained. Interveinal yellowing of leaves — when the veins are green and the tissue between the veins is yellow — is symptomatic of iron chlorosis. At a distance of several feet, chlorotic plants look very yellow.
Lowering the pH in high lime soils can reduce problems with iron chlorosis, but the simplest approach may be to apply fertilizer containing iron. Several products are available. Follow directions on the container for proper use. A manure application also can help supply iron.
Zinc deficiency occasionally occurs when soils contain excess lime, are low in organic matter or have been eroded or recently disturbed. Sweet corn and beans are the most likely crops to be affected.
If zinc deficiency is suspected, the level of zinc in the soil can be determined by a soil test. If the zinc level is low, applying two to four ounces of zinc per 1,000 square feet will correct the problem. The application of barnyard manure also is helpful. Zinc should be applied prior to tillage and can be applied as a foliar spray. To ensure an effective application, follow the directions on the container.
Other nutrients (calcium, magnesium, boron, chlorine, copper, manganese and molybdenum) needed by plants and usually supplied by the soil are not known to be deficient in Nebraska soils and usually are of less concern in producing vegetable crops; however, low calcium transport in a plant is associated with blossom end rot of tomatoes.
Adjusting Fertilizer Based on Your Garden Area
The lime, sulfur, and nutrient requirements presented in Tables I-VI are given as pounds per 1,000 sq ft. To adjust the rates according to the exact area of a garden or part of a garden use Equation 3:
Equation 3
| Pounds needed for garden area = Rate from table x (garden length in ft x garden width in ft/1,000) |
For example, an area of a garden that will be planted to carrots measuring 25 feet (length) by 5 feet (width) and has a soil phosphorus concentration of 4 ppm (see Table V) will need 0.375 lb of phosphorus [3 lb x (25 ft x 5 ft/1,000)].
Applying Materials When a Weight Scale Is Not Available
It is often convenient to apply amendments and fertilizers based on a volume such as cups or ounces per garden area where a weight scale is not available. To determine the volume of material to apply, determine the following information: total volume (cups, quarts, gallons, etc.) of material in the container, weight of material in the container (listed on container), and total amount of amendment you want to apply over the garden area. Use Equation 4 to determine the volume of material to apply to the garden area.
Equation 4
| Volume of material to apply = pounds of material to apply / (total pounds of material in container / total volume of material in container)* |
| *Do calculations in parenthesis first. |
For example, an individual needs to apply 3.1 lb of the product and determines that the 5 lb bag of fertilizer (analysis = 8-10-8) has 8 cups of material. From this information the individual calculates that approximately 5 cups of fertilizer are needed to apply 0.25 lb of nitrogen to the garden area (3.1 lb / (5 lb / 8 cups)).
Other useful Extension publications on soil fertility and fertilizers can be found at http://soilfertility.unl.edu/.
Acknowledgment
The authors have revised the original NebGuide: G945, A Gardener’s Guide for Soil and Nutrient Management in Growing Vegetables, and recognize the contributions of the previous authors, E.J. Penas and D.T. Lindgren.
Visit the University of Nebraska–Lincoln Extension Publications Web site for more publications.
Index: Horticulture
Vegetables
1990, Revised April 2005