G2329· Index: Food Science & Technology


José Rodrigo Mendoza, Post-doctoral Research Associate
Andréia Bianchini, Associate Professor
Corn (Zea mays) is a staple food in Mexico and both Central and South America with rapid growth in consumption in Europe and the United States (2, 10). This agricultural commodity is prone to fungal damage due to inadequate pre- and post-harvest handling practices, which can lead to a reduction of yield and quality. Moreover, the presence of certain molds is often accompanied by mycotoxin contamination, compromising the safety of corn. Mycotoxins are defined as secondary metabolites of filamentous fungi (molds), predominantly belonging to the genera Alternaria, Aspergillus, Claviceps, Fusarium, and Penicillium (9).
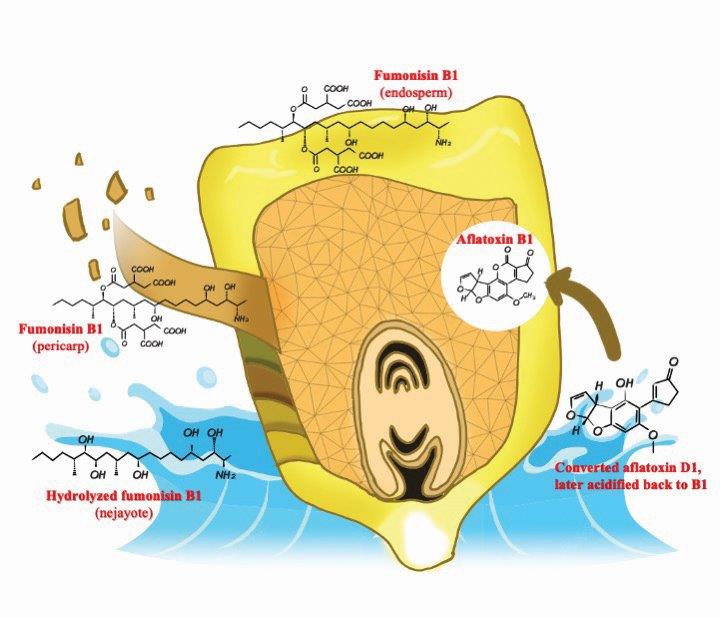
Fig. 1. Exemplification of hypothesized fate of some mycotoxins during corn nixtamalization. Some mycotoxins can be partially solubilized while in other cases they can be masked and still be present in food.
Human and animal exposure to mycotoxins occurs primarily through food and can result in acute or chronic intoxications, known as mycotoxicoses, with symptoms varying depending on dosage, length of exposure, specific toxin, and the health of the individual (31).
Controlling mycotoxins poses a challenge worldwide, being most problematic in tropical and subtropical regions. The two primary fungal toxin groups affecting corn are aflatoxins and fumonisins (3, 25). However, other toxins such as zearalenone, deoxynivalenol, citrinin, and ochratoxin have been reported in corn as well (9, 20, 24). More information regarding specific mycotoxicoses can be found in the NebGuide Understanding Fungal (Mold) Toxins (Mycotoxins), G1513.
Originating in Mesoamerica, nixtamalization is the alkaline (lime, Ca(OH)2) cooking of corn. The alkaline solution degrades and removes the pericarp, softens the endosperm structure, and allows diffusion of water and calcium ions into the inner starch portion of the kernel which may have an influence on mycotoxin contamination (Figure 1). In the traditional nixtamalization process (Figure 2), after alkaline cooking, corn kernels are steeped for approximately 24 h, and are later washed to remove the remains of the pericarp and excess calcium (8, 10, 19).
The resulting product, called nixtamal, is ground to produce the masa dough, which is the base for several products such as tortillas, tamales, corn cakes (e.g. pupusas, arepas), liquid products (e.g. atole, pozole), and snacks. Besides sensorial changes, nixtamalization enhances the nutritional value of the corn. As the calcium content is increased, niacin becomes available, and phytic acid levels are reduced (2, 27). In addition, nixtamalization can impact mycotoxin levels in the resulting food product. Effects on aflatoxins and fumonisins present in contaminated corn undergoing this alkaline thermal treatment are discussed in this publication.
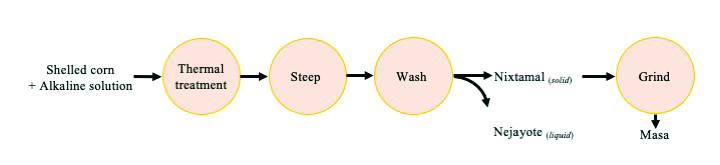
Fig. 2. Traditional nixtamalization and masa production process.

Fig. 3. Examples of aflatoxin and fumonisin chemical structures. The aflatoxin lactone ring and tricarballylic acid moieties from fumonisin are marked in red.
Fumonisins (Figure 3B) are mycotoxins produced mainly by Fusarium verticillioides and F. proliferatum. The forms generally present in contaminated food are members of the B series, fumonisin B1 (FB1), B2 (FB2), B3 (FB3), and B4 (FB4) (16). Of these, the International Agency for Research on Cancer (IARC) has classified FB1 as a Group “2B carcinogen”, indicating this toxin is possibly a carcinogenic to humans (4). Fumonisins are heat-stable (100–120°C), especially at neutral pH, and tolerate many of the commonly used thermal treatments in food processing and preparation (12, 13). Table 1 includes some findings of the effects of nixtamalization on fumonisins.
The high pH of the alkaline nixtamalization processing promotes ionization of starch hydroxyl groups, as well as the hydrolysis of the parent fumonisin molecule (6) by removing the carboxylic acid moieties. Rat-feeding bioassays have aimed to evaluate the detoxifying effectiveness of nixtamalization. In contrast to FB1, hydrolyzed FB1 (HFB1) did not cause neural tube defects (30). During food processing, fumonisins may bind to various components within the food matrix (e.g. nixtamal) or react with other ingredients such as reducing sugars. Bound mycotoxins may be an issue as they can be masked and remain undetected by conventional methods resulting in an underestimation of the potential toxicity of the contaminated products. Recent findings (4) indicate that fumonisin quantification in the steeping water (nejayote) and masa dough, during nixtamalization, accounted for more than what was initially quantified in the corn kernels, evidencing forms of matrix-associated fumonisins that were released during processing.
Fumonisins are water-soluble and nixtamalization may lower the fumonisin content of food products if the nejayote is discarded (23), thereby reducing the hepatotoxic and nephrotoxic potential of contaminated masa and derived products made from contaminated corn (28). Moreover, partially hydrolyzed FB1 (PHFB1) and HFB1 formed during nixtamalization tend to be found mainly in the cooking/steeping liquid and solid waste. Also, the amount of FB1 in the masa and tortillas tends to decrease with relative increases in lime, although boiling time has no apparent effect (29). At higher lime concentrations more pericarp is removed resulting in reduced fumonisin levels. Nixtamalization, coupled with rinsing, is crucial to reducing the presence of fumonisin in the final product, whereas grinding, sheeting, baking, and cooking the masa seems to have little impact. Loss of fumonisins throughout processing may indicate that they are extracted or otherwise removed from products, destroyed, chemically modified, bound to matrix components, or largely unextractable (11).
Aflatoxins (Figure 3A) are toxic and hepatocarcinogenic compounds produced by most of the strains of the Aspergillus parasiticus and some of the A. flavus fungi. Aflatoxins (AF) found in grains include B1, B2, G1, and G2 (16), where AFB1 is the most potent, naturally-occurring liver carcinogen known (15). Aflatoxins are resistant to thermal inactivation, with a decomposition temperature ranging from 237–306°C, although this can vary depending on water availability and pH (16, 26). It has been suggested that the presence of moisture in food matrices facilitates the opening of the lactone ring in AFB1, allowing the formation of a terminal carboxylic acid which enables its degradation via heat-induced decarboxylation (13). Alkaline conditions encourage thermal degradation through increased aflatoxin solubility (Table 2). However, the pH inside of the kernels does not increase significantly when compared to the surrounding lime solution. Therefore, it is likely that only the toxin located on the outer layers of the kernels may be solubilized into the nejayote fraction, potentially increasing the safety of the masa. The opening of the lactone ring can be, however, a reversible phenomenon. Modified aflatoxins on the masa-derived products can be converted back to their original form by acidification in the human digestive tract during digestion (23, 26). Elias-Orozco et al (7) showed that the addition of 3% hydrogen peroxide (H2O2) to the corn-water mixture resulted in a higher reduction of AFB1 than when each treatment, lime or H2O2, was applied independently. This phenomenon is possibly due to the reaction of lime with the lactone ring and the interaction between H2O2 and the double bond in the furan ring of the aflatoxin molecule. Despite the potential of this treatment this addition compromises the palatability of the masa and derived products.
Table 1. Examples of reported effects of nixtamalization processing conditions on fumonisin reduction. Parts per million (ppm) = milligrams of fumonisin per kilogram (mg/kg) of corn masa.
|
Type of fumonisin (FB) |
Process parameters |
Quantification method |
Fumonisin decrease or increase in masa |
Remarks |
Reference |
|---|---|---|---|---|---|
|
FB2, 5 ppm |
pH 10, 175–200°C, 60 min heating |
HPLC |
Up to 90% FB2 decrease |
Reduction regardless of pH 4–10. Minimum recommended processing: 150°C, 60 min. |
(12) |
|
FB1, 8.8 ppm |
pH 10, 100–125°C, 5 min steaming |
HPLC |
89.5% FB1 decrease |
Dry basis. Hydrolyzed fumonisins (HFB) were the major decomposition compounds detected. |
(6) |
|
LOD: 0.01 ppm |
8.4% HFB1 increase |
||||
|
FB1, 34.6 ppm |
Alkaline pH. Proprietary. |
HPLC, LC-MS |
86.4% FB1 decrease |
Partially hydrolyzed FB1 (PHFB1) found predominantly in the liquid and solid waste. |
(29) |
|
PHFB1, 1.34 ppm |
32.8% PHFB1 decrease |
||||
|
HFB1, 0.95 ppm |
5.5% HFB1 decrease |
||||
|
FB1, 0.7–1.65 ppm |
pH 10.6–11.1. 15–60 min boiling |
HPLC |
80–100% FB1 decrease |
FB1 decreased as lime increased. Boiling time had no apparent effect. |
(5) |
|
LOD: 0.025 ppm |
|||||
|
FB1, 38.1 ppm |
105 min boiling |
HPLC, LC-MS |
50% total fumonisins decrease |
Total FB1/HFB1 in residual lime water and water washes accounted for 50% of the total FB1 in the uncooked maize. |
(21) |
|
LOD: 0.025 ppm |
Table 2. Examples of reported effects of nixtamalization processing conditions on aflatoxin reduction. Parts per billion (ppb) = micrograms of aflatoxin per kilogram (μg/kg) of corn masa.
|
Type of aflatoxin (AF) |
Process parameters |
Quantification method |
Aflatoxin decrease or increase in masa |
Remarks |
Reference |
|---|---|---|---|---|---|
|
Total aflatoxin |
~pH 10 (nejayote) |
Fluorometry |
Traditional: 52–85% total aflatoxin decrease |
Most aflatoxin modification and detoxification occur in the cooking liquor due to high pH. |
(26) |
|
98°C |
|||||
|
Traditional: 40 min boiling |
Commercial: 30–71% total aflatoxin decrease |
||||
|
Commercial: Directly steeping |
|||||
|
Total aflatoxin, 678.3 ppb |
pH 8.24, 35 min at 85°C |
HPLC |
93.2% decrease |
Acidification caused reformation of the original aflatoxin on corn masa. |
(18) |
|
LOD: 0.5 ppb |
57.2% increase |
||||
|
Total aflatoxin, Level 1: 29 ppb, Level 2: 93 ppb |
Traditional: 0.99% lime (w/v), 70 min at 85°C |
HPLC |
Traditional: Level 1: 89% decrease, Level 2: 87% decrease |
Ecological nixtamalization uses less water and uses the whole grain. |
(17) |
|
Ecological: 0.37% lime (w/v), 10 min at 92°C |
LOD: 0.5 ppb |
Ecological: Level 1: 25% decrease, Level 2: 13% decrease |
|||
|
AFB1, 495 ppb, AFM1, 402 ppb, AFB1 dihydro-diol, 30.4 ppb |
0.3% lime (w/w), 30 min at 90–96°C |
HPLC |
94.2% AFB1 decrease |
Addition of 3% H2O2 resulted in a higher reduction of AFB1 |
(7) |
|
LOD: 5 ppb AFB1, 15 ppb total AF |
94.5% AFM1 decrease |
||||
|
92.8% AFB1 di-hydrodiol decrease |
Aside from fumonisins and aflatoxins, there are other mycotoxins that can be found in corn. Deoxynivalenol (vomitoxin, DON) and its derivatives are mycotoxins produced by certain Fusarium mold species that frequently infect corn, wheat, and other grains. DON has been reported to be significantly reduced after nixtamalization due to its instability in alkaline conditions. Similarly to what is observed with fumonisins, the outer layer of contaminated corn kernels contains a high amount of DON, therefore the removal of the pericarp during the nixtamalization process further reduces this contaminant (14). Abbas et al (1) reported that zearalenone, an estrogenic mycotoxin produced by some Fusarium species, can undergo a reduction of 59–100% in contaminated corn following nixtamalization. The same group also reported a loss of 72–82% of DON, as well as a total (100%) reduction of 15-acetyl-DON in corn samples after alkaline cooking. Reduction of moniliformin via alkaline cooking of corn has also been evaluated (22), and a 71% reduction of the toxin was observed during the nixtamalization process.
Reduction of mycotoxins in processes such as nixtamalization is variable, as it depends of several parameters not limited to cooking time, temperature, pH, and other food ingredients. During nixtamalization, both the removal of the pericarp, as well as mycotoxin solubility during steeping seem to be directly related to the contamination levels found in the final corn-based product.
While mycotoxins can potentially be controlled to some degree with nixtamalization through removal, chemical modification to less toxic compounds, or degradation, food processors are recommended to always use good quality starting materials, in accordance to regional mycotoxin regulations in place, to prevent mycotoxin contamination of corn-based products. Even though processing methods may assist in reducing contamination, ensuring the safety of corn-based products should primarily rely on the use of grain, that is as much as possible, free of mycotoxins.
1. Abbas, H. K., C. J. Mirocha, R. Rosiles, and M. Carvajal. 1988. Decomposition of zearalenone and deoxynivalenol in the process of making tortillas from corn. Cereal Chem.
2. Caballero-Briones, F., A. Iribarren, and J. Peña. 2000. Recent advances on the understanding of the nixtamalization process. Superf. y vacío 10:20–24.
3. Chulze, S. N. 2010. Strategies to reduce mycotoxin levels in maize during storage: a review. Food Addit. Contam. Part A. Chem. Anal. Control. Expo. Risk Assess. 27:651–7.
4. De Girolamo, A., V. M. T. Lattanzio, R. Schena, A. Visconti, and M. Pascale. 2016. Effect of alkaline cooking of maize on the content of fumonisins B1 and B2 and their hydrolysed forms. Food Chem. Elsevier Ltd 192:1083–1089.
5. De La Campa, R., J. D. Miller, and K. Hendricks. 2004. Fumonisin in tortillas produced in small-scale facilities and effect of traditional masa production methods on this mycotoxin. J. Agric. Food Chem. 52:4432–4437.
6. Dombrink-Kurtzman, M. A., T. J. Dvorak, M. E. Barron, and L. W. Rooney. 2000. Effect of nixtamalization (alkaline cooking) on fumonisin-contaminated corn for production of masa and tortillas. J. Agric. Food Chem. 48:5781–5787.
7. Elias-Orozco, R., A. Castellanos-Nava, M. Gaytán-Martínez, J. D. Figueroa-Cárdenas, and G. Loarca-Piña. 2002. Comparison of nixtamalization and extrusion processes for a reduction in aflatoxin content. Food Addit. Contam. 19:878–885.
8. Fernández-Muñoz, J. L., M. E. Rodríguez, R. C. Pless, H. E. Martínez-Flores, M. Leal, J. L. Martinez, and L. Baños. 2002. Changes in nixtamalized corn flour dependent on postcooking steeping time. Cereal Chem. 79:162–166.
9. Gullino, M. L., J. P. Stack, J. Fletcher, and J. D. Mumford. 2017. Practical Tools for Plant and Food Biosecurity, 8th ed. Springer, Cham, Switzerland.
10. Gutiérrez-Cortez, E., J. I. Rojas-Molina, M. L. Zambrano-Zaragoza, D. Quintanar-Guerrero, R. M. González-Reza, A. Rojas-Molina, and D. G. Espinosa-Arbeláez. 2013. Effect of processing conditions on the production of nixtamalized corn flours by the traditional method. CYTA—J. Food 11:46–53.
11. Humpf, H. U., and K. A. Voss. 2004. Effects of thermal food processing on the chemical structure and toxicity of fumonisin mycotoxins. Mol. Nutr. Food Res. 48:255–269.
12. Jackson, L. S., J. J. Hlywka, K. R. Senthil, and L. B. Bullerman. 1996. Effects of Thermal Processing on the Stability of Fumonisin B2 in an Aqueous System. J Agric Food Chem 44:1984–1987.
13. Kabak, B. 2009. The fate of mycotoxins during thermal food processing. J. Sci. Food Agric. 89:549–554.
14. Kaushik, G. 2015. Effect of Processing on Mycotoxin Content in Grains. Crit. Rev. Food Sci. Nutr. 55:1672–1683.
15. Lee, H. J., and D. Ryu. 2015. Advances in Mycotoxin Research: Public Health Perspectives. J. Food Sci. 80:T2970–T2983.
16. Magan, N., and M. Olsen. 2004. Mycotoxins in food: detection and control, 1st ed. CRC Press.
17. Méndez-Albores, J. A., G. Arámbula-Villa, M. G. Loarca-Piña, J. González-Hernández, E. Castaño-Tostado, and E. Moreno-Martínez. 2004. Aflatoxins’ fate during the nixtamalization of contaminated maize by two tortilla-making processes. J. Stored Prod. Res. 40:87–94.
18. Méndez-Albores, J. A., G. A. Villa, J. C. Del Rio-García, and E. M. Martínez. 2004. Aflatoxin-detoxification achieved with Mexican traditional nixtamalization process (MTNP) is reversible. J. Sci. Food Agric. 84:1611–1614.
19. Méndez-Montealvo, G., F. J. García-Suárez, O. Paredes-López, and L. A. Bello-Pérez. 2008. Effect of nixtamalization on morphological and rheological characteristics of maize starch. J. Cereal Sci. 48:420–425.
20. National Corn Growers Association. 2018. Mycotoxins: What is a Mycotoxin, Aflatoxin, Fumonisin. Mycotoxin Inf.
21. Palencia, E., O. Torres, W. Hagler, F. I. Meredith, L. D. Williams, and R. T. Riley. 2003. Total fumonisins are reduced in tortillas using the traditional nixtamalization method of mayan communities. J. Nutr. 133:3200–3203.
22. Pineda-Valdes, G., D. Ryu, D. S. Jackson, and L. B. Bullerman. 2002. Reduction of moniliformin during alkaline cooking of corn. Cereal Chem. 79:779–782.
23. Ryu, D., A. Bianchini, and L. B. Bullerman. 2008. Effects of processing on mycotoxins. Stewart Postharvest Rev. 4.
24. Shotwell, O. L. 1991. Natural Ocurrence of Mycotoxins in Corn, p. 325. In Mycotoxins and Animal Foods. CRC Press, Boca Raton, Florida.
25. Torres, O., J. Matute, J. Gelineau-van Waes, J. R. Maddox, S. G. Gregory, A. E. Ashley-Koch, J. L. Showker, K. A. Voss, and R. T. Riley. 2015. Human health implications from co-exposure to aflatoxins and fumonisins in maize-based foods in Latin America: Guatemala as a case study. World Mycotoxin J. 8:143–159.
26. Torres, P., M. Guzmán-Ortiz, and B. Ramírez-Wong. 2001. Revising the role of pH and thermal treatments in aflatoxin content reduction during the tortilla and deep frying processes. J. Agric. Food Chem. 49:2825–2829.
27. Valderrama-Bravo, C., A. Rojas-Molina, E. Gutiérrez-Cortez, I. Rojas-Molina, A. Oaxaca-Luna, E. De la Rosa-Rincón, and M. E. Rodríguez-García. 2010. Mechanism of calcium uptake in corn kernels during the traditional nixtamalization process: Diffusion, accumulation and percolation. J. Food Eng. Elsevier Ltd 98:126–132.
28. Voss, K. A., C. W. Bacon, F. I. Meredith, and W. P. Norred. 1996. Comparative Subchronic Toxicity Studies of Nixtamalized and Water-extracted Fusarium moniliforme Culture Material. Food Chem. Toxicol. 34:623–632.
29. Voss, K. A., S. M. Poling, F. I. Meredith, C. W. Bacon, and D. S. Saunders. 2001. Fate of fumonisins during the production of fried tortilla chips. J. Agric. Food Chem. 49:3120–3126.
30. Voss, K., D. Ryu, L. Jackson, R. Riley, and J. Gelineau-Van Waes. 2017. Reduction of Fumonisin Toxicity by Extrusion and Nixtamalization (Alkaline Cooking). J. Agric. Food Chem. 65:7088–7096.
31. Wild, C. P., and Y. Y. Gong. 2009. Mycotoxins and human disease: A largely ignored global health issue. Carcinogenesis 31:71–82.
This publication has been peer reviewed.
Nebraska Extension publications are available online at http://extensionpubs.unl.edu/.
Extension is a Division of the Institute of Agriculture and Natural Resources at the University of Nebraska–Lincoln cooperating with the Counties and the United States Department of Agriculture.
Nebraska Extension educational programs abide with the nondiscrimination policies of the University of Nebraska–Lincoln and the United States Department of Agriculture.
© 2021, The Board of Regents of the University of Nebraska on behalf of the Nebraska Extension. All rights reserved.