G2075
Downy Mildew of Sunflower in Nebraska
The causes, economic importance, symptoms, cycle, and photos of sunflower downy mildew and the fungus that causes it are included in this publication.
Robert M. Harveson, Extension Plant Pathologist
- Introduction
- Pathogen and Economic Importance
- Signs and Symptoms
- Disease Cycle and Epidemiology
- Management
Introduction
Sunflower downy mildew was first described from the northeastern United States in the late 19th century on Eupatorium, a flowering plant in the same family as sunflower (Compositae). Thus it, like the cultivated sunflower (Helianthus annuus L.), is a native of North America and the Western Hemisphere. With cultivated sunflower’s worldwide movement, the pathogen is now distributed wherever the crop is grown except for Australia. Sunflower downy mildew can cause heavy losses of up to 50-95 percent in cool wet years. Because of this destructive potential, it is one of the most serious sunflower diseases.
Pathogen and Economic Importance
Downy mildew of sunflower is caused by the obligate fungal pathogen, Plasmopara halstedii. Most downy mildews have restricted host ranges, but P. halstedii causes disease on at least 80 species in 35 genera of the family Compositae. This includes many weed and horticultural species. The pathogen can be seedborne, but that is rare; P. halstedii is more commonly associated with infecting young plants through the roots as a soilborne pathogen.
As of 2008, 12 physiological races of the pathogen were known worldwide. The occurrence of a new physiological race indicates that new cultivars containing known resistance genes are now susceptible to disease. Fortunately, the majority (more than 75 percent) of the identified pathogen species consisted of three primary races. During the 2009-2010 seasons, additional surveys of sunflower fields throughout the Great Plains revealed the presence of five additional new pathogen races. Most of these were found in North Dakota, but one was identified from a wild sunflower species growing on a ditch bank in Banner County, Nebraska (Figure 1).
Yield losses from downy mildew depend upon numerous factors, including incidence and distribution of the pathogen, percentage of infected plants in the field, and the season’s weather. Surviving plants can often compensate for those that prematurely die after early infection. However, yield losses may also become more noticeable and serious when large field areas such as low spots are affected (Figure 2). Yield losses generally are additive, the combination of plant mortality, lighter and fewer seeds produced by surviving plants, and lowered oil contents.
 |
 |
|
| Figure 1. Wild sunflower species infected with new “hot” race of downy mildew found in 2010 in Banner County, Nebraska. | Figure 2. Low spot in a field with a high incidence of the downy mildew pathogen. | |
 |
 |
|
| Figure 3. A young sunflower plant infected very early as a seedling, showing effects of systemic infection. | Figure 4. Another early-infected sunflower plant showing severe stunting. |
Signs and Symptoms
Infection at the seedling stage can occur under optimal environmental conditions, resulting in a systemic disease (Figure 3). Damping-off or seedling blight occurs when seedlings are exposed to high concentrations of inoculum in the soil combined with cool (54-57°F), very wet soils.
Downy mildew infections can be subdivided into two major categories based on symptoms. Systemic symptoms are produced when young seedlings are infected through the root system, often resulting in plant death. If seedlings survive, they are severely stunted (Figure 4) with chlorotic, puckered leaves (Figure 5). Chlorosis is an indication of the portion of leaves being colonized by the pathogen. Chlorosis will often remain associated with the leaf veins (Figure 6), but can also cover entire areas of the leaf (Figure 7). With extended periods of cool (59-65°F) weather coupled with high humidity or leaf wetness from rain or dew, a dense cottony, white growth will form on leaves, generally on the underside (Figure 8). If systemically infected plants reach maturity, they produce few if any viable seeds, and heads will characteristically face upwards (Figure 9). Head size is additionally reduced, as is both the number and weight of seeds per head. Rarely, systemic symptoms may also appear on leaves in the middle or upper leaves of the plant, with no downy mildew symptoms being observed on the lower leaves (Figure 10). Secondary infections occur when windblown zoospores from adjacent plants land on leaves of uninfected plants, provided that sufficient leaf moisture is available, but do not become systemic (Figure 11). These lesions are generally chlorotic and angular in shape (Figure 12), being limited by leaf veins. Found directly beneath yellow lesions on the underside of leaves is the white cottony sporulation of the fungus, which is diagnostic (Figure 13). Plants are susceptible to secondary infection for longer periods of time than with the systemic root infections, yet rarely result in yield loss. Both types of infection have been observed on wild sunflower species as well (Figure 14).
 |
 |
|
| Figure 5. Another early-infected sunflower plant showing chlorotic, puckered leaves. | Figure 6. Systemic infection showing chlorotic leaf veins, suggesting pathogen colonization of the vascular system. | |
 |
 |
|
| Figure 7. Systemic infection of two adjacent sunflower plants with chlorosis in association with veins and larger areas of the leaf surface. | Figure 8. White cottony growth of pathogen (sporulation) on bottom leaf surface as a result of extended periods of cool and wet weather. | |
 |
 |
|
| Figure 9. Systemically infected plant that reached maturity. Note severe stunting and movement of head upward. | Figure 10. Rare occurrence of systemic infection in middle leaves. Note lack of systemic downy mildew symptoms on lower leaves. | |
 |
 |
|
| Figure 11. Local lesion infection with lesions coalescing. Note lack of systemic movement of symptoms into newer leaves. | Figure 12. Local infections from windblown zoospores. Note the angular appearance of chlorotic lesions on upper leaf surface. | |
 |
 |
|
| Figure 13. Local infection from windblown zoospores. Note sporulation of pathogen on lower leaf surface. Each sporulating spot on the bottom is associated with the upper chlorotic lesions like those in Figure 12. | Figure 14. Local lesion (A and C) and systemic (B and D) infections on wild sunflowers. Note chlorotic upper leaf surface (C and D) and fungal sporulation emerging from lower leaf surface (A and B). |
Disease Cycle and Epidemiology
|
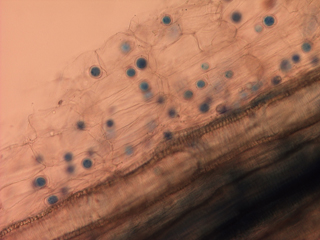
The pathogen resides in soil as sexually-produced oospores. These round-to-oblong, thick-walled oospores are formed in root tissues shortly after infection (Figure 15) and serve as survival structures. They are highly resistant to adverse environmental conditions, and are capable of remaining dormant in soils for up to 10 years. When soils are saturated and cool (54-57°F), oospores can germinate and form zoosporangia that release motile zoospores that swim through soil water. New systemic infections occur when zoospores contact and infect seedling roots. Those surviving plants will then exhibit signs and symptoms like those previously described as disease progresses (Figures 12 and 13), producing inoculum for further spread through secondary infections.
Management
- Genetic Resistance. Using resistant cultivars, where available, is the most efficient control measure for downy mildew. However the appearance of new physiological races makes this technique inconsistent, and possibly ineffective in all production systems.
- Seed Treatments. Fungicide seed treatments can be a valuable tool for this disease. However, the pathogen has developed resistance to metalaxyl and mefanoxam, two of the more common fungicides for seed treatments. Other fungicides are labeled such as Dynasty (similar product to Quadris), and likely more will eventually be available. Foliar fungicides aren’t recommended, as their use is ineffective based on the systemic nature of the disease.
- Control Weed Hosts. Since wild and volunteer sunflowers are hosts for the pathogen, eliminating these plants will help reduce overall inoculum build-up in fields.
This publication has been peer reviewed.
Disclaimer Reference to commercial products or trade names is made with the understanding that no discrimination is intended of those not mentioned and no endorsement by University of Nebraska–Lincoln Extension is implied for those mentioned. |
Visit the University of Nebraska–Lincoln Extension Publications Web site for more publications.
Index: Plant Diseases
Sunflower
Issued May 2011