G1995
Rust of Sunflower in Nebraska
This NebGuide discusses how to recognize and manage rust of sunflower in Nebraska.
Robert M. Harveson, Extension Plant Pathologist
- Introduction
- Pathogen and Disease Cycle
- Factors Favoring Disease Severity and Losses
- Management
- Conclusions and Observations from 2009
Introduction
Sunflower rust, caused by the fungal pathogen Puccinia helianthi, has traditionally been a sporadic but potentially serious disease problem in Nebraska, particularly in irrigated production. When conditions are favorable for disease development, it can cause significant losses in both yield and seed quality on susceptible hybrids.
Rust is present to some extent each year in Nebraska in both cultivated and wild sunflowers. In commercial production, however, it often occurs late enough in the season (after flowering) that yields aren’t affected and treatment isn’t necessary.
Pathogen and Disease Cycle
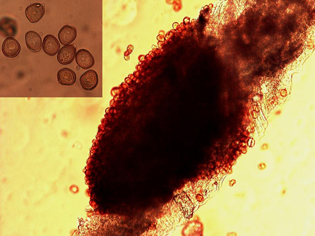
The rust pathogen has a complex life cycle consisting of five distinct spore stages — all of which occur on sunflower. The most damaging and commonly observed spore stage is the uredial stage, and is characterized by reddish-brown, cinnamon-colored pustules (uredia) found on both top and bottom leaf surfaces (Figure 1). The uredial pustules are often surrounded by yellow halos (Figure 2) and contain thousands of spores (urediospores) (Figure 3). These “rust-colored” pustules and spores are the origin for the disease name and generally develop in mid to late summer.
This stage of the cycle is also called the “repeating stage” because the spores can repeatedly infect new leaves and plants throughout the season as long as conducive disease conditions are maintained. However, as temperatures cool in the fall (less than 50°F), these spores are converted to dark, two-celled teliospores which serve as the overwintering stage of the fungus (Figure 4).
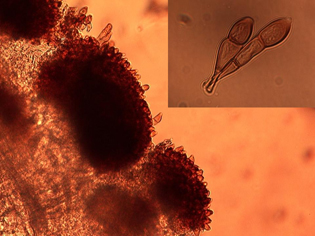
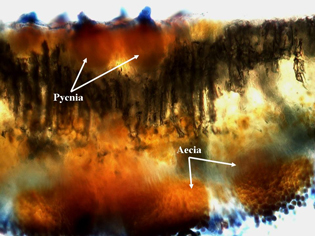
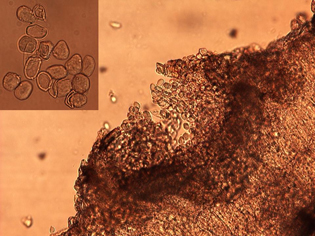
In early spring, teliospores germinate to produce basidiospores, which can infect sunflower seedlings. The basidiospore infections give rise sequentially to the pycnial and aecial spore stages. Pycnial lesions are circular and orange measuring 2 to 5 mm in diameter and surrounded by a yellow halo (Figure 5). Flask-shaped pycnia are found embedded within lesions on the upper surface (Figure 6). Aecia, which develop directly from the pycnia, are usually found on the lower leaf surface directly below the pycnia (Figure 6). The aeciospores, formed in developing aecia, (Figure 7) then re-infect sunflowers to create new uredia, completing the life cycle. The aecia are recognized as clusters of small yellowish-orange cups filled with spores (Figure 8). The entire life cycle of P. helianthi has been worked out in the lab, but the early spore stages (pycnial and aecial) of P. helianthi are seldom observed in nature.
 |
 |
|
| Figure 1. Reddish-brown to cinnamon-colored uredial pustules found on both upper (left) and lower (right) leaf surfaces. | Figure 2. Uredial pustules surrounded by yellow halos. | |
 |
 |
|
| Figure 3. Microscopic thin section from an infected sunflower leaf showing a uredial pustule producing urediospores. Close-up of single-celled urediospores (inset). | Figure 4. Microscopic thin section from an infected sunflower leaf showing a telial pustule producing teliospores. Close-up of two-celled teliospores (inset). | |
 |
 |
|
| Figure 5. Orange pycnial lesions infecting cotyledons of volunteer sunflower (first evidence of infection). | Figure 6. Microscopic thin section from an infected sunflower leaf showing flask-shaped pycnia on the top leaf surface, and aecial cups and developing aeciospores on the bottom leaf surface. | |
 |
 |
|
| Figure 7. Microscopic thin section from an infected sunflower leaf showing an aecial pustule producing aeciospores. Close-up of single-celled aeciospores (inset). | Figure 8. Yellowish-orange aecial cups on underside of infected sunflower leaf. |
Factors Favoring Disease Severity and Losses
Environmental conditions favoring infection and disease development of the urediospore stage include a minimum of two to three hours of leaf wetness originating from dew, rainfall or irrigations and maximum infections will occur after 6 to 8 hours of free water on leaves. Urediospores germinate and cause new infections at temperatures ranging from 55 to 85°F.
New infections can occur every 8-14 days depending on environmental conditions, with higher temperatures resulting in more rapid infections. For example, at 77°F, more than 90 percent of urediospores germinate if the leaf stays wet for three hours. At 68°F, the leaf must stay wet for more than eight hours to induce 90 percent germination of spores.
Once a leaf has been infected, temperature is the critical factor for continued pathogen growth and disease development, with moisture having little to no influence. With cool conditions, (65°F in day time or lower than 60°F at night), it takes approximately 14 days from time of infection until new uredial pustules appear and a second disease cycle begins. At warmer temperatures (85°F in daytime or greater than 70°F at night), new pustules are produced in as few as eight days.
Time of infection profoundly influences further disease development and eventual yield reductions. Early infection (Figure 9) obviously allows more time to produce new or repeated infection cycles. Plants that become infected prior to bloom are also more seriously affected by P. helianthi (Figures 10 and 11), due in part to a greater susceptibility of younger tissues than on maturing plants later in the season. Greenhouse studies have concluded that 40 percent yield losses are experienced in plants infected before bloom compared to 10 percent losses in those infected after bloom.
 |
 |
|
| Figure 9. Young (six- to eight-leaf) volunteers infected with uredial pustules in early June prior to the planting of any commercial sunflower fields. | Figure 10. The same volunteers from Figure 9 now beginning to die in late July. | |
 |
 |
|
| Figure 11. The same volunteers from Figure 9 now dying in mid-August. | Figure 12. Aecial cups on underside of infected wild sunflower found in a ditchbank (top). Young pycnial lesion on cotyledon of volunteer found in late May 2009 on periphery of field cropped to sunflowers in 2008 (bottom). |
Management
- To reduce the chances of early infections, destroy volunteers and wild species. This breaks the disease cycle before the uredial stage is formed.
- Since the pathogen overwinters as teliospores in infested residue, do not plant sunflowers two years in a row in the same field.
- Planting early and using short-season hybrids will generally result in fewer problems from rust.
- Resistant hybrids are available in both confection and oilseed types.
- Fungicide usage also can be effective and several fungicides are registered for use on sunflower for rust, including Headline® (pyraclostrobin), Quadris® (azoxystrobin), and Folicur® (tebuconazole). Regular scouting for the presence of rust will help.
- The economic threshold for applying fungicides is difficult to estimate. As a rule of thumb, uredial infections appearing on the upper two to three leaves closest to the head prior to petal drop will usually economically justify fungicide applications.
Conclusions and Observations from 2009
The spring of 2009 in western Nebraska’s Panhandle was characterized by unusually cool conditions with above-average precipitation. A survey of production fields cropped to sunflowers in 2008 in western Nebraska was conducted over the next four weeks (May 26 to June 19) to determine the incidence and distribution of the early spore (pycnial and aecial) stages of P. helianthi, from cultivated volunteers.
The survey monitored more than 50 locations in nine counties throughout the Panhandle, and also included wild sunflowers found in ditchbanks and disturbed areas in close proximity to fields. One of the nine counties had no identifiable pathogen. The pathogen was identified, however, from 44 out of 52 (85 percent) of surveyed fields/sites representing eight of the nine counties surveyed, including Scotts Bluff, Morrill, Box Butte, Sioux, Cheyenne, Kimball, Banner, and Duel.
The pathogen’s early spore stages (pycnia and/or aecia) were readily identified from both cultivated volunteer (Figure 12-bottom) and wild sunflower species (Figure 12-top) in 2009. The widespread presence of the early stages of rust throughout Nebraska’s sunflower production areas resulted in much earlier uredial infections than normal. The first infected commercial fields were found in late June. Thus the major impact from these early infections was the need to treat for rust in early to mid-July.
Growers in Nebraska are not accustomed to spraying for rust until mid-August, if at all. It’s not known whether the high incidence of the early spore stages of rust is a common occurrence or simply a consequence of the cool, wet environment experienced during 2009 in Nebraska, but these findings will continue to be monitored in future seasons.
This publication has been peer reviewed.
Disclaimer Reference to commercial products or trade names is made with the understanding that no discrimination is intended of those not mentioned and no endorsement by University of Nebraska–Lincoln Extension is implied for those mentioned. |
Visit the University of Nebraska–Lincoln Extension Publications Web site for more publications.
Index: Plant Disease
Sunflower
Issued February 2010