G1958
Halo Blight of Dry Beans in Nebraska
Halo blight and its management are covered in this NebGuide.
Robert M. Harveson, Extension Plant Pathologist
Introduction
Halo blight, like common bacterial blight, has been found in Nebraska for more than 70 years. It’s considered a major problem anywhere moderate temperatures occur during bean production. Central High Plains losses have been reduced by planting cultivars with genetic resistance to the pathogen. While several popular cultivars have good levels of resistance to common and halo blights, they are more prone to infection by rust or white mold.
Symptoms and Signs
The first symptoms of infection are small water-soaked spots on leaflets. Under arid conditions, the infected tissue dies and turns tan-colored and necrotic. A broad yellow-green halo then develops around necrotic spots (Figure 1), contrasted with the narrow, bright yellow border around lesions characteristic with common blight (Figure 2). The necrotic spots additionally remain very small (Figure 3), unlike that of common blight (Figure 4).
Haloes are not evident in hot weather. If infected systemically, young leaflets become curved and chlorotic and don’t show necrotic spots or broad yellow halos. Yellow-green chlorosis becomes more pronounced at temperatures of 68°F to 72°F (17°C to 20°C) due to the pathogen’s production of a toxin.
When temperatures are above 75°F (24°C), toxin production often decreases and chlorotic symptoms become less noticeable. Then the symptoms appear similar to brown spot. In severe cases, a general systemic chlorosis may develop (Figure 5). Systemic infections are not commonly encountered, but occur more readily in some dry bean market classes such as light red kidneys.
Pod symptoms begin as water-soaked circular spots (Figure 6) or streaks along pod sutures. Bacterial ooze may emerge from stems, pods or leaves seven to 10 days after infection, giving lesions a greasy, water-soaked appearance. On common isolation media, bacterial colonies are a cream to silvery color, in contrast to the yellow colonies found with the common blight bacterial pathogen. Seeds may shrivel or discolor if lesions expand into pod sutures or penetrate young pod walls.
 |
 |
|
| Figure 1. Halo blight lesions from field-grown dry beans consisting of a broad yellow-green halo surrounding necrotic center. | Figure 2. Common blight lesion in comparison consisting of a narrow lemon-yellow halo surrounding necrotic center. | |
 |
 |
|
| Figure 3. Necrotic center for halo blight normally remains very small. | Figure 4. Necrotic center for common blight normally is much larger than that of halo blight. | |
 |
 |
|
| Figure 5. Systemic halo blight infection showing overall general chlorosis and stunting of plant. | Figure 6. Small water-soaked lesions on pods characteristic of early halo blight infection. Credit: H.F. Schwartz. |
Pathogen and Disease Cycle
Pseudomonas syringae pv. phaseolicola (Psp) is an aerobic or oxygen-requiring bacterium. Psp can usually be identified from cream- to white-colored colonies on standard media (Figure 7). However, it also produces diffusible, fluorescent pigments in culture on media deficient in iron (Figure 7), and thus is also classified as a fluorescent pseudomonad, as is the brown spot pathogen. Maximum growth and production of the bacterial-induced toxin occurs at 68°F to72°F (17°C to 20°C), which is responsible for the chlorosis symptoms described above.
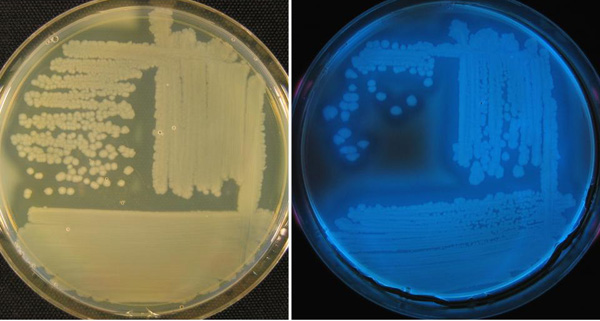 |
| Figure 7. White-cream colored growth of halo blight pathogen on standard media (left), compared with fluorescent growth on iron-deficient media under a black light (right). Credit: H.F. Schwartz. |
Halo blight is a low temperature disease and most destructive in areas where temperatures are moderate. Yield loss potential is greatest at temperatures that enhance production of the toxin (68°F to 72°F;17°C to 20°C). It’s frequently detected early in the season, shortly after its emergence on seedlings or during mid-vegetative stages of plant growth when conditions are favorable.
Like common blight, the halo blight pathogen survives in residue or seeds from the previous year. However, seed transmission these days is very low in the U.S., due to the use of western-grown certified seed grown in arid areas.
However, the pathogen is commonly found on leaves as an epiphyte (an organism that can survive on the external leaves of another plant) on legume hosts. Several pathogenic races have been identified, distinguished by host specificity, and substantial variation in virulence has been observed in natural populations.
Management
Chemical Methods
Chemical control results vary depending on pathogen, weather and disease pressure. Increased yields have resulted from treating halo blight and brown spot infections with copper-based applications 40 days after emergence (mid-vegetative or early flowering periods) and repeating application every seven to 10 days for a total of three applications.
Cultural Methods
- Do not save seed from previously blighted fields for re-use.
- Plant certified seed of disease-resistant cultivars where possible.
- Plant seed treated with streptomycin to help reduce contamination of the seed coat and establish a vigorous, early-season stand.
- Rotate beans with other crops for two to three years.
- Incorporate infected bean residues after harvest.
- Eliminate bean volunteers during growing season.
- Stay out of bean fields when wet. Moving through blighted fields when foliage is wet can spread pathogen to other plants.
- Do not spread old bean straw from infested crops on new fields to be used for bean production.
- Avoid re-using irrigation water.
- Do not plant beans close to recently blighted fields.
This publication has been peer reviewed.
Visit the University of Nebraska–Lincoln Extension Publications Web site for more publications.
Index: Plant Diseases
Dry Beans
Issued July 2009