G1740
Guidelines for Soil Sampling
Soil samples representative of a field are the best guidelines to determine fertilizer needs. This publication describes proper procedures to collect representative soil samples.
Richard B. Ferguson, Gary W. Hergert, Charles A. Shapiro and Charles S. Wortmann
Extension Soil Specialists
- Objectives
- General Guidelines
- Soil Sampling Equipment
- Time of Sampling
- Handling of Samples
- Acknowledgments
Objectives
The primary objectives of soil sampling are to determine the average nutrient status and degree of variability in a field. Correct fertilizer use, based on accurate information about soil fertility levels in fields, can result in increased crop yield, reduced cost and minimized environmental impact. Knowing a field’s nutrient status variability means fertilizer application can be adjusted to more closely meet the supplemental nutrient needs of a crop for specific field areas.
|
General Guidelines
Determine Sampling Approach
With the development of technologies and procedures for site-specific management of fertilizer and other inputs, producers can collect and quantify information about soil nutrient variability within a field. Prior to sampling, decide how soil nutrient information will be used to manage fertilizer, and that will help determine how samples should be collected. For uniform fertilizer application, collect soil samples randomly within representative areas of the field. If variable rate fertilizer application is anticipated, sample either in predefined management zones or in a grid pattern with known sample locations.
Uniform Fertilizer Application
If fertilizer is to be applied uniformly, it still is helpful to have some idea of the variability in soil fertility within a field. Knowing this variability may allow you to adjust rates, application timing or fertilizer sources accordingly. Collect samples from subareas within fields that are relatively uniform. These areas can be determined based on soil type, slope, degree of erosion, cropping history, known crop growth differences, spatial patterns of crop yield and any other factors that may influence nutrient levels in the soil.
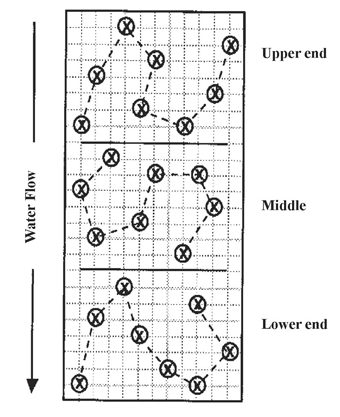
Avoid odd areas in the field (eroded spots, turn rows, abandoned farmsteads or feedlots), or sample them separately. Soil samples from these areas can significantly alter test results for the rest of the field. When sampling furrow-irrigated fields for residual nitrate-nitrogen, collect samples from the upper, middle and lower portions of the field (Figure 1). The amount of irrigation water that infiltrates the soil will influence the amount and depth of nitrate-nitrogen in the soil.
Variable Rate Fertilizer Application
There are two basic approaches to soil sampling for site-specific fertilizer management — grid sampling or management zone-based sampling. Both approaches provide more detailed information about the variability of nutrient levels within a field than sampling normally done as described above for uniform fertilization. Grid sampling is more expensive and time-consumin≠g, but can provide useful information for variable rate fertilization for several years. Management zone sampling is based on zones derived from various spatial information resources — yield maps, soil surveys, aerial photographs, soil apparent electrical conductivity, etc. Often information from several spatial data layers can be combined to derive management zones. Figure 2 illustrates grid and management zone approaches to sampling a field. More detailed information on site-specific sampling is available in two other resources — Soil Sampling for Precision Agriculture (EC154) and Site-Specific Nitrogen Management for Irrigated Corn (EC163).
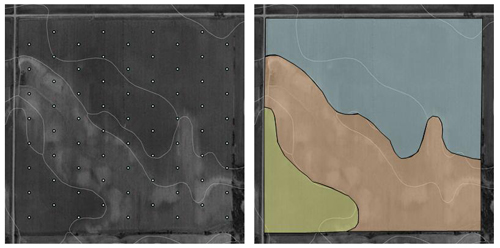 |
| Figure 2. Examples of grid and management zone approaches to collecting soil samples. Figure 2a has 72 sample points. Within each of the three management zones in Figure 2b, 10-15 cores should be collected and composited into a sample representing each zone. |
Select Proper Sampling Depth
Surface samples are used to determine soil pH, lime need, organic matter, phosphorus, potassium, sulfur and zinc. In Nebraska, soil test correlation and calibrations for these tests are based on surface samples collected from 0-8 inches. It is important to use the same sampling depth when re-sampling fields so soil test values over time can be accurately compared. Sampling deeper than 8 inches generally results in lower test values for organic matter, phosphorus and zinc. Potassium and pH may increase, decrease or remain the same with deeper samples. Surface samples are needed for all crops. Fertilizer recommendations for all nutrients except nitrogen are based on nutrient levels in the surface soil sample. Nitrogen recommendations for many crops depend on the organic matter content in the surface soil sample, as well as residual nitrate-nitrogen in surface and subsurface samples.
Stratification of soil nutrients can occur when fields have not been tilled for several years, with higher nutrient concentrations close to the soil surface, often in the top 2-3 inches. Availability of nutrients from fields where stratification exists generally is not a concern, as plant roots can effectively access nutrients at shallow depths. However, it is important to sample to the proper depth of 8 inches, with complete mixing of all cores collected prior to retention of a subsample to send to the lab. If stratification exists and samples are not collected to the proper depth or not well mixed, there is greater risk of a nonrepresentative sample and an inaccurate fertilizer recommendation.
Both surface (0-8 inches) and subsurface (below 8 inches) samples are needed to accurately estimate nitrate-nitrogen in the root zone, because nitrogen in the nitrate form moves easily with water and will leach into the subsoil. Nitrate-nitrogen in the root zone is readily used by plants. For most soils and annual crops, roots will reach a depth of 4 feet or more. To accurately predict nitrate-nitrogen in the root zone, subsurface samples should be collected to a depth of 3 feet. A 2-foot sample is the minimum sampling depth recommended for nitrate-nitrogen, and will not predict plant available nitrate-nitrogen as accurately as a deeper sample. For crops with shallow root zones, such as dry beans, canola and millet, a 2-foot sample is adequate. If rooting depth is limited because of coarse sand or gravel, rock or a high water table, sample to the depth possible. Nitrogen fertilizer recommendations for several crops grown in Nebraska are based on the amount of nitrate-nitrogen in the root zone determined from subsurface samples, as well as organic matter content in the surface sample. If subsurface samples for nitrate-nitrogen arenít taken, nitrogen recommendations for crops will be based on historical average values of nitrate-nitrogen in the root zone, and the accuracy of fertilizer recommendations may decrease.
Collect Soil Cores
A soil core is an individual sample collected at one spot in the field. For each area of the field to be sampled, collect cores randomly throughout the area, unless information is being collected for site-specific fertilizer management. Take care to adequately represent the entire area when sampling. Be sure to sample the entire 0-8 inch layer for general fertility analysis. Place individual soil cores in a clean plastic pail for mixing. Separate pails should be used for subsurface samples. Break up and thoroughly mix soil cores in each pail after collecting samples over the entire area. After mixing, retain a portion of the mixed soil and place it in a properly labeled sample bag or box to send to the laboratory for analysis. Typically, a sample of a pint volume, or one pound in weight, will be adequate for analysis. The sample label should include the producer’s name, field ID, sample ID, and depth of sample (Figure 3).
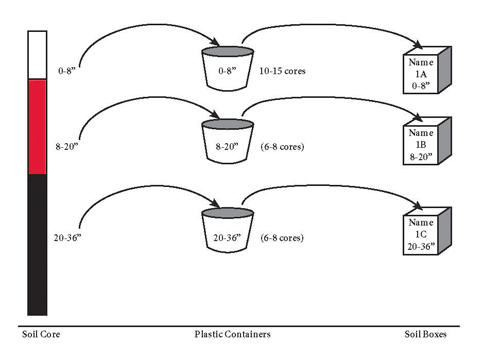 |
| Figure 3. Division of soil cores by depth, with retention of a well-mixed subsample into labeled boxes or sample bags. |
|
The University of Nebraska–Lincoln recommends that samples represent fields or areas within fields no larger than 40 acres. Larger areas may contain enough variability in soil properties and nutrient values to render the average soil test level from a single sample meaningless. Sampling field areas smaller than 40 acres in size can increase the accuracy of the test, and provide a measure of variability across the field.
Acceptable measurement of the average nutrient status in a 40-acre area can be obtained with 10 to 15 randomly collected surface cores and six to eight subsoil cores for nitrate-nitrogen analysis. For furrow-irrigated fields, four to five subsurface cores per 20 acres generally will provide more useful estimates of nitrate-nitrogen than six to eight cores per 40 acres, provided the field is divided into upper, middle and lower portions based on the direction of water flow across the field.
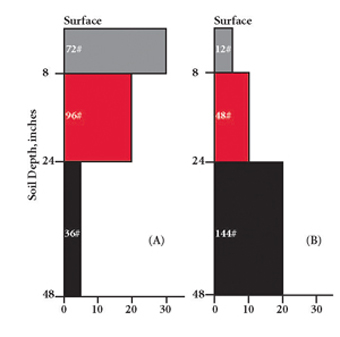
Subsurface samples should be continuous to the bottom of the core. For example, with a surface sample of 0-8 inches, collect the subsurface sample from 8-36 inches. However, information about the vertical distribution of nitrate-nitrogen in the field can be obtained if the subsoil sample is broken into segments. A surface sample of 0-8 inches, combined with a subsoil sample separated into depth increments of 8-20 and 20-36 inches, has several advantages over a single subsurface sample. It is difficult to obtain a well-mixed, representative sample from multiple cores covering a large depth range. Variations in soil texture and moisture by depth, coupled with the large volume of soil involved, make mixing difficult. Also, nitrate-nitrogen concentration in the subsoil is likely to vary with depth. The normal pattern is for nitrate-nitrogen concentrations to decrease with depth, but that is not always the case. If nitrate-nitrogen concentrations increase at deeper depths, perhaps caused by dry growing conditions followed by improved moisture and increased crop nitrogen removal, the availability of nitrate-nitrogen in the subsoil may be overestimated. Figure 4 illustrates two situations where the total amount of root zone nitrate-nitrogen is the same. Figure 4a is typical. Figure 4b has a significant amount of nitrate-nitrogen deeper in the root zone, which may result in the deeper nitrate-nitrogen leaching below the root zone before crop roots can reach it. For situations like that in Figure 4b, it is appropriate to increase nitrogen fertilizer rate recommendations because of uncertainty regarding availability of nitrate-nitrogen deep in the root zone.
Soil Sampling Equipment
Surface soil samples can be collected using a soil probe or soil auger. The soil probe is the most desirable tool for collecting soil samples. It will give a continuous core with minimal disturbance of the soil. Cores can be subdivided for various depth increments. In many soils, a probe can be placed back into the hole left by sampling the surface layer to collect a subsoil sample. Normally very little contamination occurs from one depth to another with a soil probe. A soil probe cannot be used when the soil is too wet, too dry, rocky or frozen. High clay content soils can be difficult to sample with a probe, but most problems can be avoided by using a tip intended for high clay soils; avoiding very wet or dry conditions; lubricating the probe with silicone spray; and using a probe that is in good condition.
A soil auger can be used in soils that are frozen or contain gravel; however, care must be taken to obtain representative samples and to avoid mixing soil from different depths. If soils are too wet or dry when sampled with an auger, mixing soil from different depths can occur. A soil auger will not effectively gather dry, powdery soils. Use a soil auger only if a soil probe cannot be used or is unavailable.
A variety of hydraulic or mechanical samplers are available for collecting both surface and subsurface samples. Generally these are designed to push soil probes into the soil, but some may have rotary heads allowing the use of an auger. For commercial use or when sampling many fields, these samplers can be very helpful.
Time of Sampling
Late fall or early winter is a good time for soil sampling, except for testing nitrate-nitrogen on coarse-textured soils. Fall sampling allows more time to get results back from the laboratory and to use the information in designing the fertilizer management program for the following year.
Fall samples should provide meaningful results for all nutrients. However, excessive precipitation between the time of sampling and when crops are grown the next year may result in some leaching of nitrate-nitrogen — either deeper in the root zone, or out of the root zone altogether. If more than 8 inches of effective precipitation (total amount that percolates into the soil) occurs on fine-textured soils, or 4 inches on coarse-textured soils, between the time of sampling and the time the crop is planted, leaching losses of nitrate-nitrogen may have occurred. If leaching loss of nitrate-nitrogen in the root zone is suspected due to winter or spring precipitation, re-sample the field.
Spring sampling prior to planting is the preferred option. Delaying sampling until spring allows soil moisture in the root zone to be replenished, thus easing sampling on many soils. The distribution of nitrate-nitrogen in the subsoil is more likely to be representative of conditions during the growing season with spring sampling.
Handling of Samples
Be careful to avoid contamination when collecting soil samples. Use clean sampling equipment and plastic buckets to receive and mix soil samples. Do not leave samples moist and warm for more than 24 hours after collection. If moist soil samples are stored for extended periods of time, additional mineralization from soil organic matter can occur, increasing soil nitrate concentrations, and perhaps affecting other nutrients as well. If samples cannot be taken to the lab within 24 hours after collection, they should be dried, refrigerated or frozen. Dry soil samples by spreading them out to air dry at room temperature for two to three days, depending on air circulation and humidity. Do not dry soil samples at high temperatures, as this can affect the analysis. Avoid contaminating samples while drying, such as with wind-blown dust. Refrigerating or freezing samples will slow or stop microbial activity adequately until the samples can be dried and ground at the lab.
Acknowledgments
The authors acknowledge the contributions to this publication by Kenneth D. Frank and Edwin J. Penas, former Extension Soil Specialists, and Richard A. Wiese, former Extension Environmental Nitrogen Specialist.
Visit the University of Nebraska–Lincoln Extension Publications Web site for more publications.
Index: Soil Management
Fertility
Issued August 2007