G1504
Lime Use for Soil Acidity Management
Soil acidity can reduce crop production by directly affecting roots and changing the availability of essential nutrients and toxic elements. Liming can neutralize soil acidity, but several factors can affect the economic benefits of liming.
Martha Mamo, Soil Scientist
Charles S. Wortmann, Nutrient Management Specialist
Charles A. Shapiro, Soil Scientist-Crop Nutrition
- Determining Lime Need
- Lime Quality and Materials
- Lime Application Considerations
- Summary
- Additional Resources
|
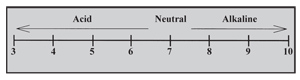
Most field crops perform best at a soil pH between 6.0 and 6.8. This pH range provides the best balance of available nutrients. When soil pH is below this range (Figure 1), some nutrients become less available (i.e., phosphorus, molybdenum). Some elements, such as manganese and aluminum, become toxic in highly acid soils (< 5.0). With continuous cropping, soil pH can decrease (i.e., increase in acidity) because of various factors, including crop removal and leaching of basic cations, application of ammonia-based nitrogen fertilizers, and organic matter decomposition. Adding lime or other materials can raise soil pH to the ideal range for crop production, create an environment for a healthy function of microbes, and increase the levels of calcium or magnesium ions.
Determining Lime Need
Soil acidity consists of active and reserve acidity. Most of the acid-causing elements (hydrogen and aluminum) are held by the cation exchange sites of the soil particles and organic matter. This is referred to as reserve acidity. Soils with large amounts of clay and organic matter have high potential for reserve acidity. Soil pH is a measure of active acidity, the hydrogen ion concentration in the soil solution. The higher the concentration of hydrogen ions in soil solution, the lower the pH (i.e., greater acidity). The active acidity is present in the immediate environment of roots and microbes. The total acidity is the sum of the reserve and active acidity. Lime neutralizes both the active acidity and some of the reserve acidity. As active acidity is neutralized by the lime, reserve acidity is released into the soil solution, maintaining the active acidity or the pH. The ability of a soil to resist changes in pH is called buffering capacity and is largely due to the reserve acidity. More lime is required to neutralize acidity on a highly buffered soil compared to a less buffered soil (Table I).
| Table I. Examples of approximate lime required to raise the pH of soils of different textural classes. (Source: Nutrient Management for Agronomic Crops in Nebraska, EC155, UNL Extension.) | ||||
Soil texture |
CEC (meq/100 g) |
Soil pH |
Buffer pH |
Lime rate (tons/acre) |
| Loamy sand Silt loam Silty clay loam |
6 14 24 |
5.6 5.5 5.6 |
6.8 6.6 6.2 |
1 2 4 |
University of Nebraska lime recommendations are based on raising soil pH to 6.5. When soil pH is less than 6.3, laboratories measure pH in a buffer solution that accounts for both active and reserve acidity. (Refer to NebGuide G1503 for more details.) Buffer solution is composed of an acid and its salt, and can neutralize both high and low pH soils. The two types of buffer solutions used in Nebraska are the Woodruff and SMP, both at pH 7.0. Soils with a pH of less than 6.3 are added to the buffer solution and the pH of the soil-buffer mix is measured. The more the soil-buffer mix pH decreases below 7.0, the higher the reserve acidity and lime requirement of the soil. The Woodruff and SMP buffer solutions give similar results for most soils; however, the Woodruff buffer is preferred for sandy soils, and the SMP buffer is preferred when the soil is high in exchangeable aluminum.
University of Nebraska lime recommendations are based on liming material that has a 60 percent effective calcium carbonate equivalent (ECCE). Effective calcium carbonate equivalent is further discussed in the Lime Quality section below. For each 0.1 pH buffer reading below 7.0, application of 1000 to 1200 lb/A of ag-lime (60 percent ECCE) is recommended to raise the soil pH to approximately 6.5 in the top 7 inches.
If lime ECCE is more or less than 60 percent, the rate is adjusted by multiplying the recommended rate by 60 and dividing by the actual ECCE (Table II). For example, if the recommended rate is 6,000 lbs (3 tons) per acre and the lime is 45 percent ECCE, then the lime rate is adjusted as:
Adjusted lime rate = Recommended lime rate x Adj. factor
6000 lb lime/A x 60 / 45 = 8000 lbs/A
| Table II. Rate adjustment for ECCE different than 60 percent. New application rate is determined by multiplying rate at 60 percent ECCE by the adjustment factor. | |
ECCE |
Adjustment Factor |
15 25 35 45 55 65 75 85 95 |
4.0 2.4 1.7 1.3 1.1 0.95 0.80 0.70 0.63 |
Lime Quality and Materials
Lime Quality
Two factors determine the effectiveness (ECCE) of liming materials:
- neutralizing value or purity, also referred to as calcium carbonate equivalent (CCE)
- particle size or fineness of the liming material.
The neutralizing value, or CCE, is the amount of acid on a weight basis that a given quantity of lime will neutralize when dissolved. It is expressed as a percentage of the neutralizing value of pure calcium carbonate or calcite (100 percent CCE). A lime that neutralizes 80 percent as much acid as pure calcium carbonate is said to have a CCE of 80. Table III shows the CCE of different liming materials.
| Table III. Calcium carbonate equivalent (CCE) of liming materials. | |
Material % |
CCE* |
|
100 75-100 75-109 120-136 179 90-95 43-44 30-70 |
| *These values only consider the purity of the material, however, the fineness also must be considered to determine the effectiveness of the
lime (i.e., ECCE = CCE times fineness). **Based on UNL research on ash from power plants in Nebraska. Fly ash CCE values and other chemical analyses should be done due to variation caused by source of coal, collection procedures and other factors. |
|
|
Particle fineness is important for lime effectiveness. The neutralization effect is greater with small particles because of increased total surface area exposed to the soil acidity. Lime distribution in the soil also is important because the lime effect of a particle extends only about 1/8 inch. Two sieves, 8 and 60 mesh, are used to separate a sample into three particle sizes (Figure 2):
a. less than 60 mesh — fine;
b. less than 8 mesh but greater than 60 mesh — medium;
c. greater than 8 mesh — coarse.
The percentages of these three components are multiplied by factors of 1.0, 0.4, and 0.1 respectively, and added together to give the fineness factor. For example, if a liming agent has a particle size distribution of 66 percent fine, 22 percent medium, and 12 percent coarse, the particle fineness of the material is calculated as:
Fineness = (66 x 1) + (22 x 0.4) + (12 x 0.1) = 76 percent
Effective calcium carbonate equivalent (ECCE) is the measure of the effectiveness of liming materials and is calculated as the product of the purity value (CCE) and the fineness value divided by 100. For example, if the purity is 80 percent and the fineness value is 75 percent, then:
ECCE = (80 x 75)/100 = 60%.
Liming Materials (See Table III)
Ground limestone is the most common liming material and consists of calcium carbonate and magnesium carbonate.
Hydrated and burned (quick) limes are quick acting and have high ECCE, but are caustic and difficult to handle.
Pel-lime (granular lime) is finely ground lime material compressed into pellets or granules to reduce dust associated with very fine particle size. The pellets dissolve in water and the particles quickly disperse and neutralize soil acidity. Application rates are less than with ag-lime because the particles are finer.
Lime slurries, also called fluid lime and liquid lime, are pulverized limestone suspended with 30 percent to 50 percent water.
Sugar factory lime is very finely ground calcium carbonate used in the production of sugar from beets.
Basic slag or calcium silicate is a byproduct of the steel industry.
Fly ash is a byproduct of coal combustion. The chemical characteristics of fly ash depend on the source of the coal. Some coals have high sulfur content and can produce fly ash with low pH while others have lower sulfur content and have high calcium and magnesium contents.
Lime Application Considerations
Lime Application
Lime takes time to neutralize soil acidity. Often as much as six months may be needed before pH changes significantly. Neutralization will be quicker if particle size is small (less than 60 mesh) and the lime is well mixed with the soil. Typically, it will take two to three years to observe the full effect of ag-lime application on soil pH.
Lime recommendations are usually made to reach a target pH in the top 7 inches of soil. If the soil is tilled to a depth greater than 7 inches, proportionately more lime is required to reach the same target soil pH. When tillage depths are reduced, lime application rates should be reduced proportionately as shown in Table IV.
| Table IV. Adjustment of lime rates for different depths of incorporation. | |
Depth of incorporation (inches) |
Factor to multiply by recommended lime rate |
2 4 6 8 10 |
0.30 0.60 0.90 1.20 1.50 |
Under no-till systems, lime is surface applied and not mixed with the soil. Mixing eventually will occur because of lime falling into cracks, earthworm activity, soil disturbance with planting and other field operations, and irrigation and/or precipitation moving the lime slowly downward. The effect of surface-applied lime in a no-till system has been found to move downward at about 1/2 inch per year on fine-textured soils. Several years are required to neutralize acidity below a 2-inch depth. Therefore, lime rates should be adjusted to 30 percent of the full rate since only the surface 2 to 3 inches of soil will be reacting with the lime. Periodic soil sampling in the 0-2, 2-4, and 4-8 inch ranges is the most reliable method to determine pH changes and lime requirement over time for no-till systems.
Cropping Systems and pH Threshold
The economic threshold for lime application depends on the most sensitive/responsive crop in the rotation. Soil pH thresholds for profitable response to lime application over a 5- to 10-year period are pH 6.0 for alfalfa-corn-soybean system; 5.6 to 5.8 for corn-soybean system, and 5.0 to 5.2 for continuous corn system. The pH thresholds are for the top 8 inches of soil with the assumption that the subsoil/ subsurface soil pH is 6.0 or greater than 6.0. (Acidification of the 8 to 24 inch subsoil is less common in Nebraska soils.)
Stratification of Soil pH
Soil pH stratification in the surface 8-inch depth should be considered when liming. Stratification of pH occurs especially in no-till sandy soils where anhydrous ammonia has been injected at a 4- to 8-inch depth for many years. At the depth of injection, an acidified layer is created due to hydrogen ions generated during the nitrification process (see NebGuide G1503).
This layer of acidity is difficult to correct under no-till systems because of slow movement of surface-applied lime. A single deep tillage to incorporate lime in the layer of acidity may be needed to alleviate the acidity problem. While there will be an added cost for the tillage operation and the loss of some of the no-till benefits, this may be more than offset by gains in productivity if a very acidic layer has developed. Sampling in layers of 0 to 2, 2 to 4, and 4 to 8 inches will help determine if tillage for lime incorporation is needed.
Site-specific or Variable Rate Application
Lime requirements vary within fields and can be mapped using grid soil sampling or, alternatively, by sampling zones within fields. These sampling zones may be determined based on crop and soil management history, variation in yield and differences in soils and landscape positions.
Variations in lime requirement may occur, depending on past practices in the field, such as: land use and cropping system (e.g., some areas of the field were cropped, while the rest may have been under pasture); manure application; nitrogen fertilizer use; and irrigation.
Topographic position can be useful in delineating sampling zones within a field. For example, on rolling cropland in southeast Nebraska, the average lime need was 21 percent more on hilltops than on hillsides and 16 percent less on bottomland than on hillsides. Soil type also can be useful for further development of sampling zones. For example, hillside soils of southeast Nebraska can be grouped into three categories: Morrill and Burchard generally need less lime; Adair, Crete, Malmo, Marshall, Otoe, Sharpsburg and Yutan have intermediate lime needs; and Nora, Geary, Pawnee, Moody and Wymore generally need more lime.
After sampling by grids, zones, or soil types, the results of the soil analysis need to be considered to determine if there is sufficient variation in lime requirement to justify variable rate or site specific application.
Economic Considerations
The cost of liming soil to a depth of 6 to 8 inches should be considered an investment of five to 10 years. This is illustrated with an example from Washington County in a disk-tilled system where the initial soil pH was 5.5 and the cost of liming with ag-lime (60 percent ECCE) was $44 per acre (Nebraska Soybean and Feed Grains Profitability Project, Peterson and Hilgenkamp). Over eight years, the total yield increase was 15 bu/A for soybeans and 8 bu/A for corn (Figure 3). Assuming soybean and corn prices of $5.60 and $2.31 per bushel respectively, an initial liming cost of $44 per acre, and an interest rate of 5 percent, the average annual income was greater than the average annual expense by year four. In this case, 80 percent of the increase came from increased soybean yield and only 20 percent from increased corn yield.
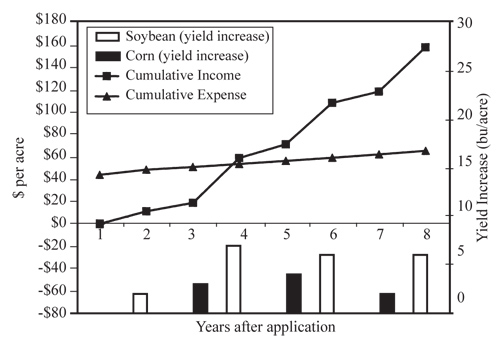 |
| Figure 3. Cumulative lime effect with tillage (initial pH of 5.5; liming cost of $44/A). (From J. Peterson and R. Hilgenkamp, Nebraska Soybean and Feed Grains Profitability Project, Washington County 2002.) |
The economics of lime use on rented land needs special consideration. Using the Washington County data, producers cannot afford to pay for lime application if the lease is less than five years. In some leases, the landowner may need to pay part or all of the cost of liming the field. Some leases stipulate that if a producer loses the lease, the landowner has to repay a portion of the producer’s investment in lime. From Washington County examples, the return realized was 10 percent, 17 percent, 55 percent and 65 percent of the lime expense in years one, two, three and four, respectively. If the lease were to be terminated after four years, the tenant would have lost 35 percent of the lime expense. The framework for expected returns of liming will need to be considered when negotiating responsibility for the cost of lime application.
Pel-lime is expected to neutralize acidity sooner than ag-lime but the long-term effectiveness of the two products in neutralizing acidity depends on their ECCE. Applying 1500 lb/A of 60 percent ECCE ag-lime eventually will have the same effect on soil acidity as 1000 lb/A of 90 percent ECCE pel-lime. Pel-lime may have a special role in some situations, such as short-term neutralization of acidity in a band near the roots of soybean to improve nitrogen fixation and yield. Surface application of pel-lime to increase pH at the soil surface may improve the performance of specific herbicides. Cost difference, however, is a major consideration when choosing between pel-lime and ag-lime.
Current recommendations are to apply enough lime to raise soil pH to about 6.5. This is well above the economic threshold for pH-induced yield loss for most Nebraska crops. Applying, for example, less than the recommended rate should be sufficient to maintain soil pH above the economic threshold for more than five years. A second application can be made several years later when soil pH again approaches the threshold level. The economic advantage of split application of lime depends on the reduction in interest cost compared to the cost of split-application. The lime source proximity and transportation costs also must be considered with a split application.
Summary
Several factors need to be considered for profitable lime use:
- Zonation of fields based on differences in management history, soil texture, soil type and topographic position should be considered in sampling for lime requirements.
- Threshold pH levels will differ for various crop rotations.
- Optimal liming practices differ for no-till and tilled conditions.
- It may take five to 10 years after application to recover the cost of liming.
- Product cost relative to ECCE is the major factor when comparing liming materials.
- Split application of a recommended amount of lime, with the second application several years later, may be more economical than applying all at once.
Additional Resources
Wortmann, C., M. Mamo, and C. Shapiro. Management Strategies to Reduce the Rate of Soil Acidification, 2003. NebGuide G1503. University of Nebraska Extension, Lincoln, NE.
Comfort, S. and K. Frank. pH and Liming. 2000. p 51-58. In R. Ferguson (ed) Nutrient Management for Agronomic Crops in Nebraska EC155. University of Nebraska Extension, Lincoln, NE.
Rehm, G., R. Monter, C. Rosen, and M. Schmitt. Lime Needs in Minnesota FO-05956-GO, 1992. University of Minnesota Extension Service, St. Paul, MN.
Vagts, T. Nitrogen Fertilizers and Soil pH. Iowa State University Web page: http://www.extension.iastate.edu/nwcrops/fertilizer_and_soil_ph.htm. 2003.
Peterson, J. and R. Hilgenkamp. 2002. Nebraska Soybean and Feed Grain Profitability Project. http://farmresearch.unl.edu. University of Nebraska Extension, Lincoln, NE.
This publication has been peer reviewed.
Visit the University of Nebraska–Lincoln Extension Publications Web site for more publications.
Index: Soil Management
Fertility
Issued March 2003, Reviewed March 2009