Michael P. Carlson, Ph.D., Emeritus Assistant Professor of Practice
Connor Biehler, M.S., Beef Assistant Extension Educator
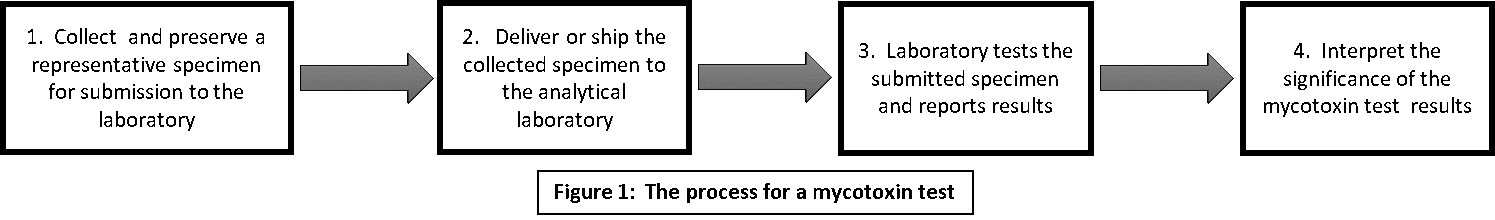
Fig. 1
The purpose of this Extension Circular is to provide information to animal owners about sampling and analyzing feed for mycotoxins.
Determining the presence or absence of mycotoxins in feed and feed components can help grain, feed, and animal producers minimize the risk of adverse health effects in animals who consume mycotoxin-contaminated feed. Chemical tests detect the presence of mycotoxins in feeds. Such tests are available at many commercial, veterinary medical and governmental laboratories.
Mycotoxins are chemicals toxic to animals and humans produced by molds infecting plants, especially grain, used for food or feed. Mycotoxins commonly found in grain or feeds used in Nebraska are aflatoxins, ergot alkaloids, fumonisins, vomitoxin and zearalenone. The Extension Circular Understanding Mycotoxins, EC3068 contains information about health effects caused by those mycotoxins and about the diagnosis and treatment of the diseases caused by them. The Extension Circular Use of Mycotoxin-Contaminated Feed for Animals, EC3066 contains information about how to use mycotoxin-contaminated animal feed if that is necessary.
Mycotoxin testing is a multi-stage process, in which you play a role. Figure 1 depicts the process.
Collection of specimens for mycotoxin testing is your or your representative’s responsibility and is a very important step in the testing process. The specimens come from the feed or feed components you are using or want to use. Hereafter in this document the term “feed” will mean feed or feed components. The challenge is to collect a specimen that adequately represents that feed.
Five to ten pounds of feed are typically collected and submitted to the laboratory for testing. Only 0.022 to 0.11 pounds (10 to 50 grams) of the submitted feed, called the analytical sample, are actually tested.
Therefore, the goal is to collect and submit a specimen that adequately represents the feed, which is critical because we assume the mycotoxin concentration found in the analytical specimen represents the concentration in the feed.
Mycotoxins are unevenly distributed throughout the feed, which makes it challenging to collect a representative specimen. There may be pockets of varying sizes containing mycotoxins in different amounts scattered unpredictably in the feed. If those pockets are missed during specimen collection, a false-negative report will result, indicating the feed is mycotoxin-free. Alternatively, a specimen collected from a contaminated pocket will make it seem that all the feed contains mycotoxins at the detected level.
Given the irregular distribution of mycotoxins in feed, the varying amounts of feeds to be sampled, and the variety of feed, how might a representative specimen be collected for submission?
Because molds produce mycotoxins, it seems logical to collect a specimen from visibly moldy areas of the feed. However, visibly moldy areas may not contain detectable mycotoxins and visibly mold-free areas may contain high concentrations of mycotoxins.
Mycotoxicoses are the diseases caused by mycotoxins.
For cases of suspected mycotoxicoses, your goal is to collect a specimen that represents the feed that caused the illness. That can be challenging because not only are mycotoxins irregularly distributed in feeds, the time between exposure to mycotoxins and observable signs of illness may range from hours to days or weeks. Collect the specimen from the feed eaten by the affected animals as soon as possible after discovery of the illness.
Collect multiple sub-samples weighing about 0.5 pounds from the entire length, depth, and width of feed bunks or feeders from which the animals were eating and combine them until you have a specimen weighing about 5 pounds. If needed, collect multiple specimens, each for separate mycotoxin testing. For example, collect and submit a specimen from each bunk, if multiple bunks were used.
Follow guidelines for collection of specimens from bulk feed if specimens from the feed used to fill the bunk/troughs or to formulate the ration are required.
Amounts of feed to be sampled can range between pounds and tons, and they may be stored in any of a variety of containers, such as bags, bins, truck beds, trailers, silos, or pit silos. Additionally, their densities and moisture contents may vary. The objective is to collect safely a representative specimen from as many parts of the feedstock as reasonably possible.
Sampling from large or bulk feed storage containers can be dangerous. Ideally, use people trained and qualified to collect samples from such storage containers. If that is not possible, some general safety considerations based on USDA grain inspection recommendations follow.
Safety procedures must consider the environment in which the sampling will occur, including but not limited to, vehicular traffic in the area, presence of utility cables or pipes, dust or feed spills in or near the storage facility, and the size and type of the feed storage container.
Means to access the feed in the storage container is another consideration. Using augers, stepladders or ladders, walkways, and climbing onto or into the bulk storage container will dictate safety measures.
Clothing
Stepladders, ladders and walkways
Sampling in a confined space
Sampling theory says to collect about 0.5 pound per 500 pounds of feed. All parts of the feed should have an equal chance of being included in the specimen. The practicality of that depends upon the amount of feed and the size, design, and number of storage containers. It also depends upon whether the feedstock is static or dynamic. It is static if it is stored as bales or stacks, or in sacks, trailers, truck beds, bins, or silos. It is dynamic if it is accessible while moving by auger or conveyor belt from one location to another.
It is easier to collect samples while the feed is moving by auger or conveyor belt from one location to another. When sampling from the moving stream, periodically collect small samples from the entire volume of the moving stream, ideally from start to finish of the movement. The sampling time for the entire lot of feed will depend upon its amount and the rate of flow during movement. If a very large amount of feed is collected, thoroughly mix it and remove a representative subsample of suitable weight for submission. Alternatively, divide the collected feed into several specimens for individual testing, which will increase the cost of testing.
If the feed is static, collect subsamples using hay or feed probes from as much of the feed as possible. The probe should be as long as possible to penetrate deeply into the feed container. Contact your local Extension office to request rental-free hay probes.
Use a probing pattern that considers the size, design, and number of storage containers. It should allow as much of the available feed to be sampled as is practical. If the lot is stored in several containers, take subsamples from each of the several containers and then combine them into one specimen. If the amount collected is too much to submit as one specimen, then thoroughly mix the collection and take a representative subsample from it for submission. Alternatively, divide the collected feed into several separate specimens for individual testing, which will increase the cost of testing.
Practically, submitted specimens usually weigh 5 to 10 pounds. The density and moisture content will determine the volume of the feed that weighs 5 to 10 pounds.
Preserve the specimen to prevent changes in mycotoxin content from occurring after specimen collection. Mycotoxin content in dry specimens is more stable than in wet specimens. Mold growth may occur in wet specimens, with subsequent mycotoxin production possible.
Generally, specimens with moisture content of no more than 13% store well. Dry wet specimens, if practical. If not, freeze the wet specimen.
Deliver or ship the specimen as early in the workweek as possible. Dry specimens are more easily shipped than wet specimens. If wet specimens cannot or should not be dried, then freeze and package them for shipment so that they remain frozen until they arrive at the lab.
Avoid shipping on days when specimen will be in transit over weekends or holiday periods, which can delay delivery. The longer the time interval between shipment and arrival at the lab, the more likely changes in specimen mycotoxin content will occur.
Consult with laboratory staff about packaging, delivery and shipment options.
Here are some factors to consider when choosing a laboratory.
Tell the laboratory what you need to have tested and for what purpose so the laboratory can determine whether any of its tests meet your need. If you are uncertain about what should be tested or what test you need, consult with laboratory staff. Here are some examples of what you or your representative might tell a laboratory.
Laboratory accreditation indicates the laboratory is competent to conduct tests and provide accurate results. Independent, third-party agencies accredit laboratories by comparing laboratory operations against a laboratory standard. The standard covers all aspects of laboratory operations, including organization; management; quality assurance; data and records management, confidentiality and security; staff competence; adequacy of facilities and equipment; test methods used; specimen receipt and preservation procedures; and reports. The standard tells laboratories what is required, but not how to meet the requirements; how a laboratory meets the requirements is up to the laboratory. Each laboratory determines which accrediting agency or agencies best suit its business and clientele.
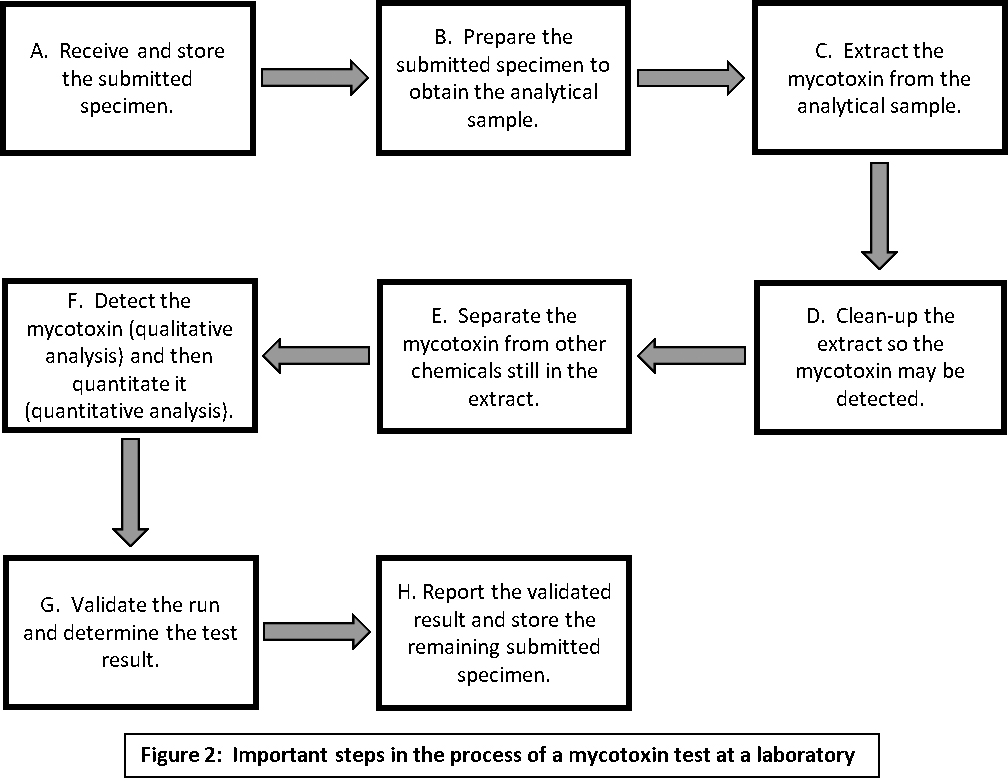
Fig. 2
Many laboratories list their accreditation(s) on their websites.
You will likely encounter one of two accreditations for laboratories offering mycotoxin tests. Veterinary medical diagnostic laboratories are accredited by the American Association of Veterinary Laboratory Diagnosticians, Inc. (AAVLD) using “Requirements for an Accredited Veterinary Medical Diagnostic Laboratory,” which it publishes. Laboratories run by various organizations may be accredited under provisions of the international standard for testing and calibration laboratories, ISO/IEC 17025. The American Association for Laboratory Accreditation (A2LA) accredits laboratories in the United States using that standard.
Laboratory testing, illustrated in figure 2, is a multistep process. It begins with the receipt of your specimen at the laboratory and ends with its report of results. The technical details of each step are beyond the scope of this document, but comments about some of the steps, beginning with Step B, might help you select one test from among several mycotoxin tests offered by a laboratory.
Test methods are designed to produce one of three types of results: qualitative, semi-quantitative, or quantitative. Qualitative methods detect the presence of a mycotoxin, but not its amount; they are sometimes called “screening methods.” Semi-quantitative methods detect the presence of a mycotoxin with some indication of its amount. For example, a semi-quantitative method for aflatoxin in corn detects aflatoxin present in concentrations either below or above 20 parts per billion (ppb), but not how much below or above 20 ppb. Quantitative methods detect the presence of a chemical and determine its concentration. For example, a quantitative method for aflatoxin in corn detects and quantitates aflatoxin at concentrations from 10 ppb to 100 ppb.
|
Unit of concentration (abbreviations) |
Definition |
Remarks |
|---|---|---|
|
Part per billion (ppb, ng/g, μg/kg, or ug/kg) |
1 part of a mycotoxin in 1,000,000,000 parts of feed |
1 ppb equals 1 billionth of a pound of mycotoxin in a pound of feed; 1000 ppb = 1 ppm |
|
Part per million (ppm, μg/g, ug/g, or mg/kg) |
1 part of a mycotoxin in 1,000,000 parts of feed |
1 ppm equals 1 millionth of a pound of mycotoxin in a pound of feed; 1 ppm = 1000 ppb |
Generally, qualitative methods are cheaper than semi-quantitative methods, and semi-quantitative methods are cheaper than quantitative. Laboratories often offer several mycotoxin tests at different prices. Request a method that meets your defined needs; see “Define your purpose and what you need tested” above. Laboratory representatives can help you choose the best test for your purpose.
To save money, sometimes a laboratory may recommend that you start with a screening method. If that test finds no mycotoxin present, then further testing is unnecessary. If it finds a mycotoxin and you need some sort of quantitative result, then another semi-quantitative or quantitative test will follow. How much, if any, money you save depends upon the number of positive specimens that need follow-up testing.
Mycotoxins are usually present in relatively small amounts measured in units of parts per billion (ppb) or parts per million (ppm). Those small amounts can significantly affect the health of animals. Table 1 provides information that can help you understand what those units mean.
Semi-quantitative and quantitative results consist of two equally important parts, a number and a unit of concentration. The number tells how many “units of concentration” are present. Simply providing the numerical part can lead to confusion. For example, if aflatoxin is said to be present at a concentration of “30,” it makes a big difference if the result is 30 parts per million (ppm) or 30 parts per billion (ppb).
Laboratories often offer tests designed to detect only one mycotoxin and other tests designed to detect multiple mycotoxins. Single-mycotoxin tests generally cost less than multi-mycotoxin tests, but the total cost of single tests for each mycotoxin included in a multi-mycotoxin test is usually greater than for the multi-mycotoxin test. Single- and multi-mycotoxin tests may be designed to screen for or quantitate mycotoxins.
No method regardless of its result type, can detect the complete absence of a mycotoxin in a specimen. Every method has a method detection limit (MDL), sometimes called a limit-of-detection (LOD) or detection limit (DL), and if a mycotoxin is present below that limit, the method cannot detect it. Consequently, results for specimens in which no mycotoxins are detected should be reported as “no detectable amount,” or “negative to test,” or some other suitable phrase that does not indicate the complete absence of mycotoxins. The limit of detection is often included in the result, for example “No detectable amount (< 1 ppb).” If a laboratory reports the absence of a mycotoxin as “0” (the number zero) with no units or detection limit, ask the reporting laboratory for the units and its test MDL.
The MDL is an important consideration when choosing a mycotoxin test. For example, if you must know if a mycotoxin is present at concentrations of 1 ppm or greater and the test MDL is 5 ppm, that test does not meet your need.
Some laboratories may provide a quantitative method’s limit-of-quantitation (LOQ) in addition to or instead of an MDL. The LOQ is the lowest reliably quantitated concentration and is greater than the MDL.
We want accurate test results that report what mycotoxin is present and how much is in a sample. Determining the accuracy of a result is challenging because we do not know what really is there nor how much.
There are two aspects to the assessment of test accuracy. One is the inherent accuracy achievable by the test, which is determined during the process of test validation. The other is the accuracy achieved every time a batch of samples is tested, which is determined using quality control measures that are part of the test protocol.
Laboratories should use validated tests. Test validation usually involves several laboratories using the same method to test multiple specimens, each containing a known mycotoxin present within a range of concentrations. Accuracy of the test is determined by comparing all test results against the known mycotoxin concentration for each specimen. Test validation also helps determine other test performance indicators, such as the MLDs, LOQs, precision, and repeatability of results. Precision and repeatability indicate how closely results for the same specimen match each time the test is run.
Sometimes test results are inaccurate, which happens for various reasons. Two important inaccuracies that may occur are false positive and false negative results. False positive results are those that detect the presence of a mycotoxin that is actually not present in the analytical specimen. False negative results are those that do not detect a mycotoxin in the analytical specimen that is actually present at a concentration above the test MDL. Method validation helps determine the incidences of false positive and false negative results for a test.
Some laboratories develop and validate their own tests. Others use tests validated or approved by government regulatory agencies or reputable technical organizations. One regulatory agency that approves mycotoxin tests is the U.S. Federal Grain Inspection Service (FGIS); FGIS-approved tests are often those validated by another organization, such as the Cereal and Grain Association, formerly the American Association of Cereal Chemists (AACC), or AOAC International, formerly the Association of Official Analytical Chemists (AOAC).
Some laboratories list information about the test method used in their catalogs of services.
Another way laboratories can check the accuracies they achieve using a test is by participating in check-sample programs. Laboratories usually pay fees to participate. Those programs provide participating laboratories with specimens containing known amounts of mycotoxins. Participating laboratories test the check samples and compare their results to the known amounts. Laboratories producing erroneous results can take corrective actions to improve their test accuracies. One example of such a program is the Mycotoxin Contamination Scheme of the Association of American Feed Control Officials (AAFCO). Laboratories that enroll in it receive four times a year animal feed or pet food samples contaminated with aflatoxins, fumonisins, vomitoxin, zearalenone, ochratoxin A, or T-2 toxin. Participating laboratories compare their results to the known mycotoxin concentrations for the check samples. If their results are inaccurate, laboratories determine why and make necessary corrections.
Laboratories may list on their websites check-sample programs in which they participate.
Select mycotoxin tests considering how certain the identity of a detected mycotoxin must be. For example, very certain identification may be necessary for mycotoxins detected in specimens involved in litigation cases.
The certainty of identification for detected mycotoxins depends upon the method of detection. Usually, the more certain the identity needs to be, the more expensive the test.
The least certain identification is “presumptive positive,” which means the chemical detected is presumed to be the mycotoxin of interest. An example of such a test is the bright greenish yellow fluorescence (BGYF) test, also called the black light test, for aflatoxins in corn. Specimens that are positive for aflatoxin by that black light test usually are analyzed using another test capable of more definitive aflatoxin identification. Presumptive positive test results are usually rapidly available and are relatively inexpensive. However, any additional testing done to confirm the identity of the mycotoxin adds to the cost.
Mass spectroscopy provides the most certain identification, and some mycotoxin tests use it as part of their mycotoxin detection process. Mass spectroscopy, which occurs in a mass spectrometer, involves breaking molecules apart by various means and then determining the masses of the molecular fragments produced. Fragmentation patterns are characteristic of specific molecules. If the fragmentation pattern of a chemical that enters the mass spectrometer matches the pattern of a known mycotoxin, that chemical is that mycotoxin. Mycotoxin tests that include mass spectroscopy usually cost more than those that do not include that method of detection.
Laboratories offer mold culture tests designed to find and identify molds present in a feed specimen. Results of those tests usually include the genus and sometimes the species of any detected molds. The detection and identification of molds present in a specimen provides limited information about mycotoxins for the following reasons.
The presence of molds in feed does not mean mycotoxins are also present. Molds are usually present in feed, including molds that may produce mycotoxins; finding no molds in a feed specimen rarely occurs.
The identification of a genus or species of mold capable of producing mycotoxins does not mean mycotoxins are present in the feed. Most mold culturing service do not differentiate between mycotoxigenic and non-mycotoxigenic strains of a mold. Mycotoxigenic strains can produce mycotoxins; non-mycotoxigenic strains cannot. However, the presence of a mycotoxigenic mold increases the chances the feed may be contaminated with mycotoxins.
Mold counts are essentially meaningless with respect to mycotoxin production because mold counts do not correlate with the presence of absence of mycotoxins in feed.
|
Service |
Method |
Specimen(s) |
Advantages |
Disadvantages |
Relative cost |
|---|---|---|---|---|---|
|
Mold culture & ID |
Fungal culture & identification |
Grains, mixed feed, feed components |
Determines the presence of molds in the submitted specimen |
Finding molds is not unusual. Generally, they do not determine mycotoxigenic potential of the cultured mold(s) |
$$ |
|
Mold count |
Plating |
Grains, mixed feed, feed components |
None for mycotoxin risk assessment or diagnostic purposes |
No correlation between mold count and mycotoxin presence or amount |
$$ (may be included with a mold culture & ID) |
|
Mycotoxin detection |
Bright greenish-yellow fluorescence (BGYF) test for aflatoxins (black-light test) |
Whole or cracked corn |
Rapid, inexpensive test |
Limited to corn, presumptive positive results |
$$ |
|
Enzyme-linked immunosorbent assay (ELISA) |
Grains, some mixed feeds, feed components |
Rapid, relatively inexpensive, good specificity; qualitative, semi-quantitative, or quantitative results |
Specific for one mycotoxin, confirmation may require additional testing |
$$ |
|
|
Test strips |
Grains, possibly some mixed feeds |
Usable on site, does not require laboratory equipment; fast results; relatively inexpensive; qualitative, semi-quantitative, or quantitative results |
Specific for one mycotoxin, confirmation may require additional testing, strips have expiration date |
$$ |
|
|
Thin layer chromatography |
Grains, mixed feeds, feed components |
Capable of multi-mycotoxin detection; qualitative, semi-quantitative, or quantitative results |
Slow, more labor intensive than other methods, confirmation may require additional testing |
$$ |
|
|
Mycotoxin detection (continued) |
High-performance liquid chromatography (HPLC) |
Grains, mixed feeds, feed components |
Capable of single or multi-mycotoxin detection; qualitative, semi-quantitative, or quantitative results |
Capabilities and cost depend upon detection method used with it, confirmation may require additional testing |
$$—$$$ |
|
HPLC-mass spec (MS), HPLC-MS-MS |
Grains, mixed feeds, feed components |
Capable of multi-mycotoxin detection; definitive identification; qualitative, semi-quantitative, or quantitative results |
Expensive |
$$$ |
|
|
Gas chromatography (GC) |
Grains, mixed feed, feed components |
Capable of multi-mycotoxin detection, qualitative; semi-quantitative, or quantitative results |
Capabilities and cost depend upon mycotoxin and detection method used with it; confirmation may require additional testing |
$$—$$$ |
|
|
GC -mass spec (MS) |
Grains, mixed feed, feed components |
Capable of multi-mycotoxin detection; definitive identification; qualitative, semi-quantitative, or quantitative results |
Expensive; not useful for some mycotoxins |
$$$ |
It is best to determine the presence or absence of mycotoxins in feeds by chemical analysis.
Table 2 lists the types of mycotoxin tests commonly used today. It lists their advantages, disadvantages, and relative costs.
Determine who can help you interpret the test results. Consider the four examples listed in the section titled “Define your purpose and what you need tested.”
If mycotoxin presence in feed is uncertain, have it tested. If you decide to collect a specimen for testing, make sure that the sample represents the feed in question. Collect and pool several small subsamples from the entire depth, width, and length of the bunks, bin, or feed pile in question. “Hot spots” could be present throughout the feed and increasing the area from which the subsamples are collected provides a better representative specimen.
If sampling from a large pile or bin, have someone collect it who is trained and qualified take the sample. If you collect the specimen yourself, make sure to use proper protective gear and safety measures.
In cases of animal illness, collect a specimen from the feed actually eaten by the ill animals as soon as possible after the illness is detected.
After the sample is collected, preserve and ship it as soon as possible, to reduce the chance that its mycotoxin content will change. Freezing wet specimens will reduce changes in the specimen. When selecting a lab to test your samples, make sure you are familiar with the tests they run and their laboratory accreditations. After receiving results, work with your veterinarian and nutritionist to discuss any potential changes that need to be made in ration formulation or feed allocation.
American Association of Veterinary Laboratory Diagnosticians, Inc. (2021) “Requirements for an Accredited Veterinary Medical Diagnostic laboratory, AC1, Version 2021–01, accessed November 2022 at https://www.aavld.org/accreditation-requirements-page.
Krska, R. and others, 2008, “Mycotoxin analysis: An update,” Food Additives and Contaminants Vol 25, No. 2, pages 152–163, https://doi.org/10.1080/02652030701765723.
Sing, J. & A. Mehta, 2020, “Rapid and sensitive detection of mycotoxin by advanced and emerging analytical methods: A review,” Food Science & Nutrition, Vol 8, pages 2183–2204.
USDA Agricultural Marketing Service, 2020, Grain Inspection Handbook, Book 1—Sampling, accessed November 2020 at https://www.ams.usda.gov/sites/default/files/media/Book1.pdf.
Wagner, C., 2015 “Critical practicalities in Sampling for Mycotoxins in Feed,” Journal of AOAC International, Vol 98, No. 2, pages 301–308.
Whitaker, T.B., 2004 “Chapter 4. Sampling for Mycotoxins” in N. Magan & M. Olsen (Editors), Mycotoxins in food Detection and control, CRC Press, Boca Raton, FL.
This publication has been peer reviewed.
Nebraska Extension publications are available online at http://extensionpubs.unl.edu/.
Extension is a Division of the Institute of Agriculture and Natural Resources at the University of Nebraska–Lincoln cooperating with the Counties and the United States Department of Agriculture.
Nebraska Extension educational programs abide with the nondiscrimination policies of the University of Nebraska–Lincoln and the United States Department of Agriculture.
© 2023, The Board of Regents of the University of Nebraska on behalf of the Nebraska Extension. All rights reserved.