EC3063


Wheat streak mosaic (WSM) was first recognized in Nebraska in 1922 as “yellow mosaic”. In the 1950s, serious epidemics occurred across the Great Plains (Figure 1), and wheat streak mosaic virus (WSMV) was identified as the causal agent. Also, during the 1950s, the only known vector for WSMV was found to be the wheat curl mite, Aceria tosichella (K.) (Figure 2). Since this first virus-mite relationship was recognized, the wheat curl mite has been found to transmit two more viruses to wheat in North America. In the 1990s, High Plains wheat mosaic virus (HPWMoV) was found to be widely distributed across the region, and in the mid-2000s Triticum mosaic virus (TriMV) was also found to be prevalent across the Great Plains. Surveys have found that these viruses occur in all Great Plains states, and co-infection with more than one virus is common. In this publication, the three viruses will be collectively referred to as the WSMV complex, of which WSMV has been shown to be the most prevalent across the region.
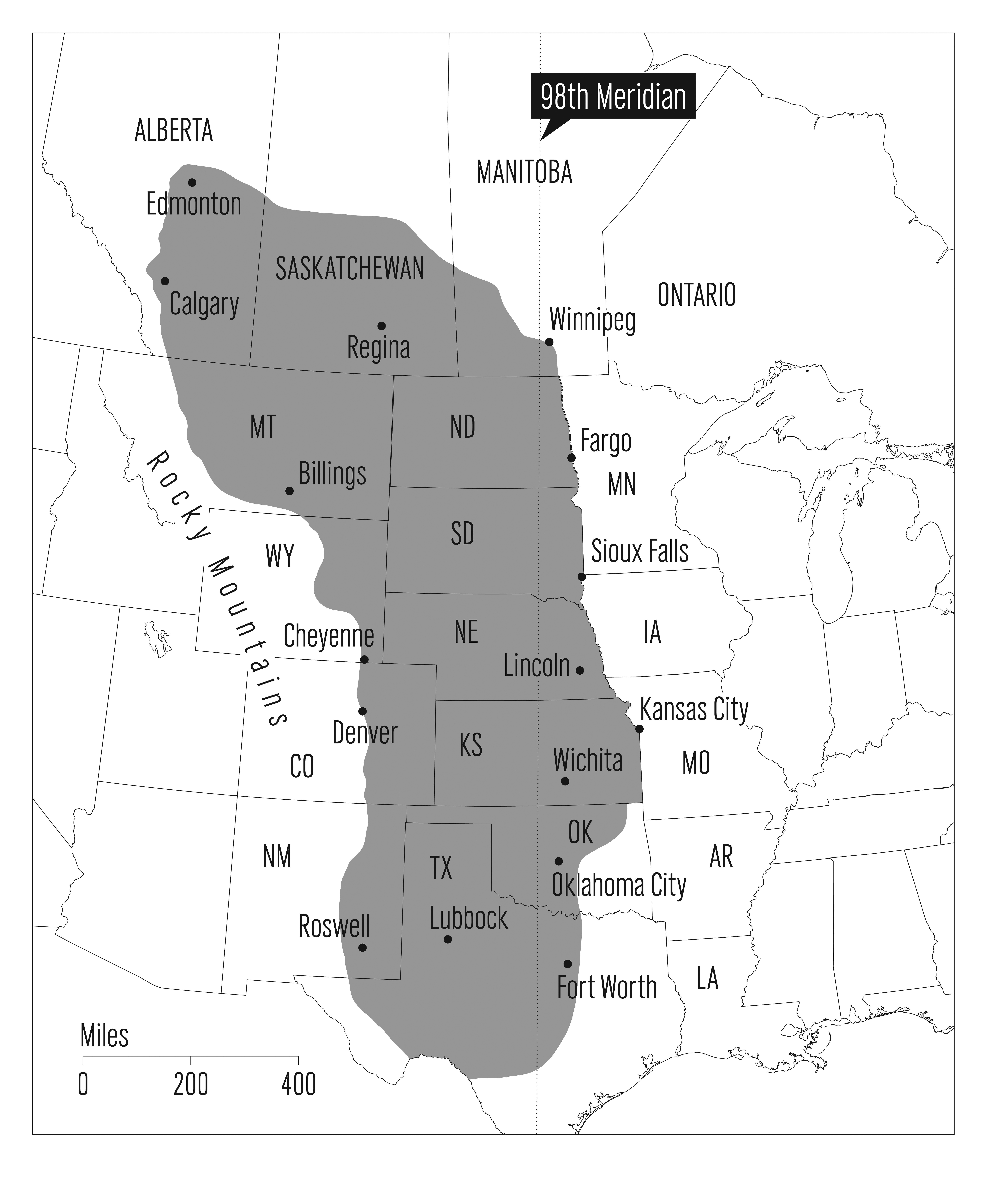
All three viruses have the potential to cause serious crop losses in wheat, but losses have been shown to be more pronounced when wheat plants are co-infected with more than one virus. In Kansas, 20-year yield loss averages associated with the WSMV complex are estimated at about 1.3% per year; however, these losses vary widely from year to year. In 2017, when widespread epidemics occurred across the Great Plains, the WSMV complex was the second most important disease in Kansas (second to stripe rust), with yield loss estimated at 5.6%. One factor that increases the impact on farmers is that individual field losses commonly reach 100% due to the WSMV complex. Losses result from a reduction in grain and forage yield. Additional losses result from increased management costs (e.g., irrigation, fertilizers, herbicides) applied to an infected wheat crop with diminishing yield potential through the spring. As with most plant viruses, a major component of effective management of the WSM disease complex relies on managing the wheat curl mite vector.
Fig. 1. The Great Plains. Source: University of Nebraska-Lincoln Center for Great Plains Studies.
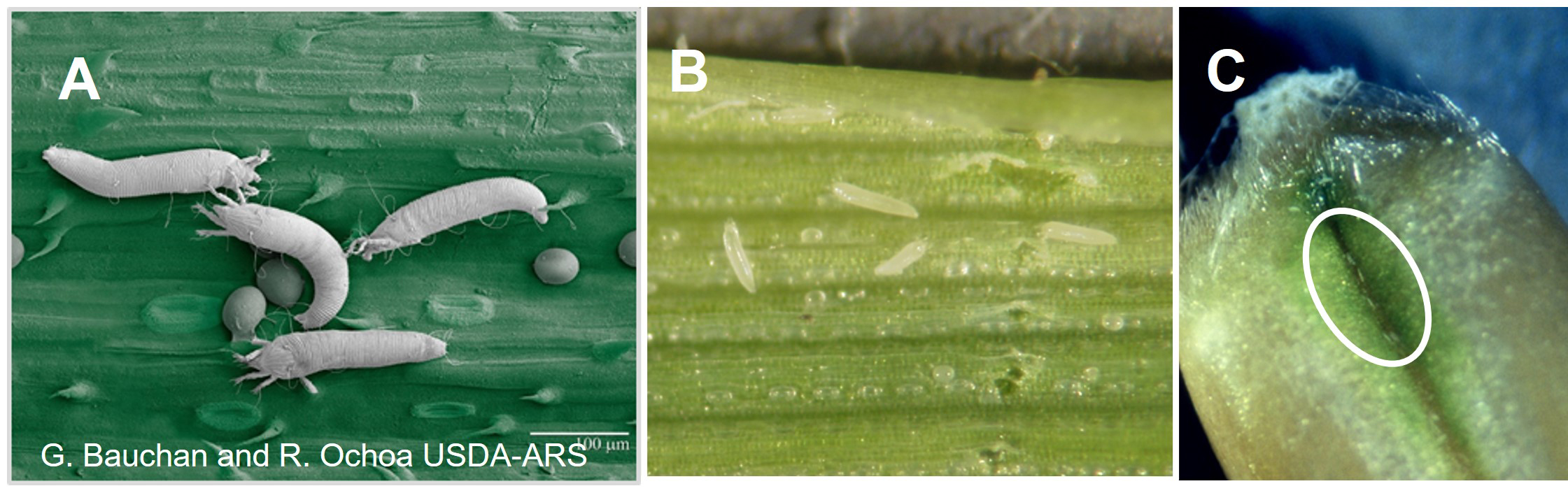
Fig. 2. Wheat curl mites and eggs on a wheat leaf (A, B), and mites on a maturing wheat kernel (C).
Leaves infested with wheat curl mites appear erect with their edges tightly rolled toward the midrib. The tip of a new leaf can be trapped in the rolled leaf below it, forming a loop (Figure 3).
Symptoms caused by the three viruses are indistinguishable, but severity increases when co-infections occur. On young leaves, symptoms start as light green streaks that elongate to form discontinuous yellow to pale green stripes, forming a mosaic pattern running parallel to the leaf veins (Figure 4).
As symptoms progress on the plant, the extent of yellowing increases on the older leaves. Symptoms are often difficult to diagnose as they can be easily confused with nutritional disorders, environmental effects, or chemical damage. Symptoms associated with other diseases such as barley yellow dwarf or wheat soilborne mosaic can mask those caused by the WSMV complex. Under severe infection, plants will be stunted, yellow, less upright than healthy plants (prostrate), and poorly tillered (Figure 5). Such plants may produce poorly filled, shriveled kernels or are entirely barren.
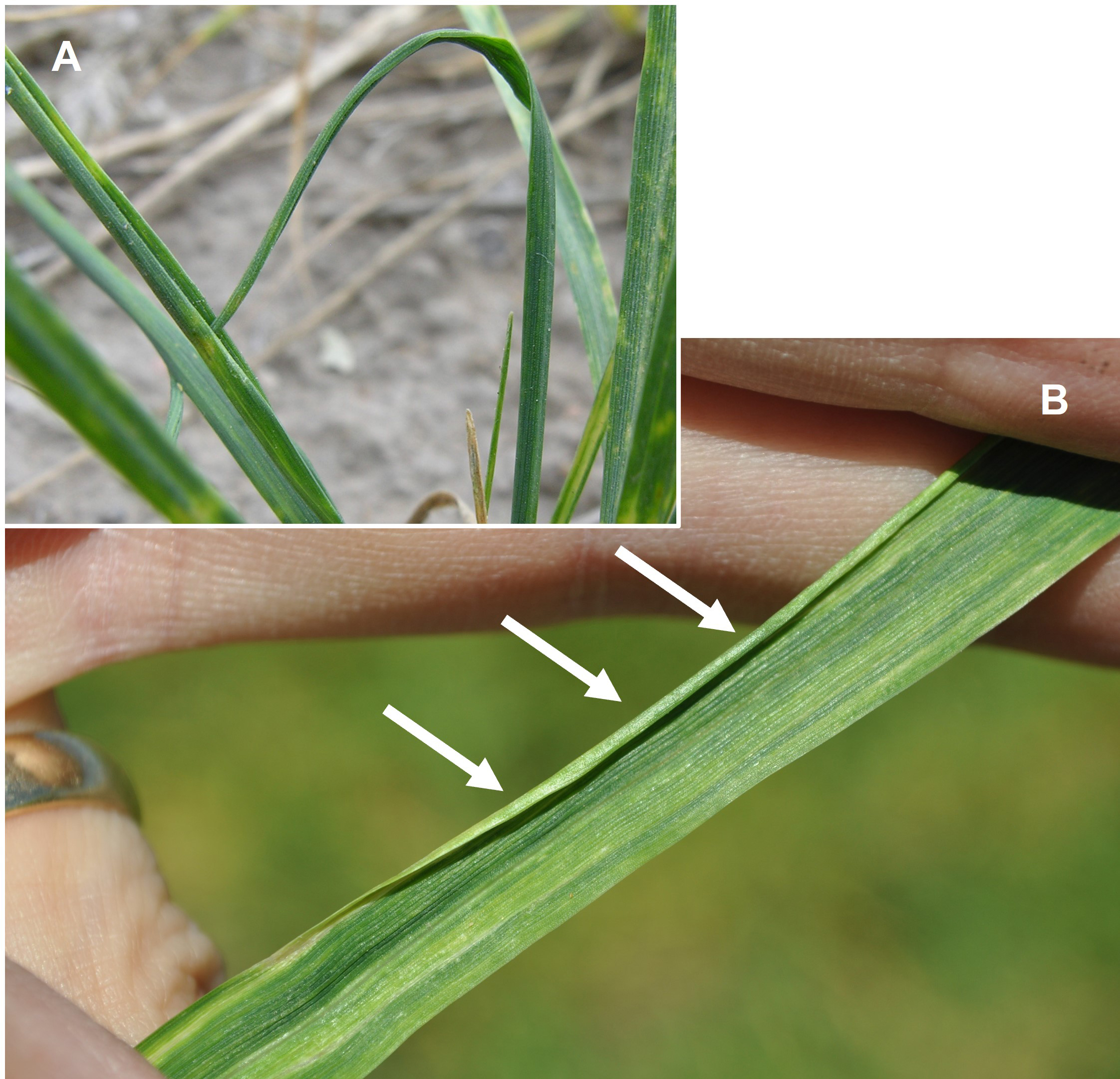
Fig. 3. Infestation of wheat curl mites on wheat results in tightly curled leaves and entrapment of subsequent leaves within the curl (A). After full leaf emergence, a tight curl at the leaf edge remains (B)
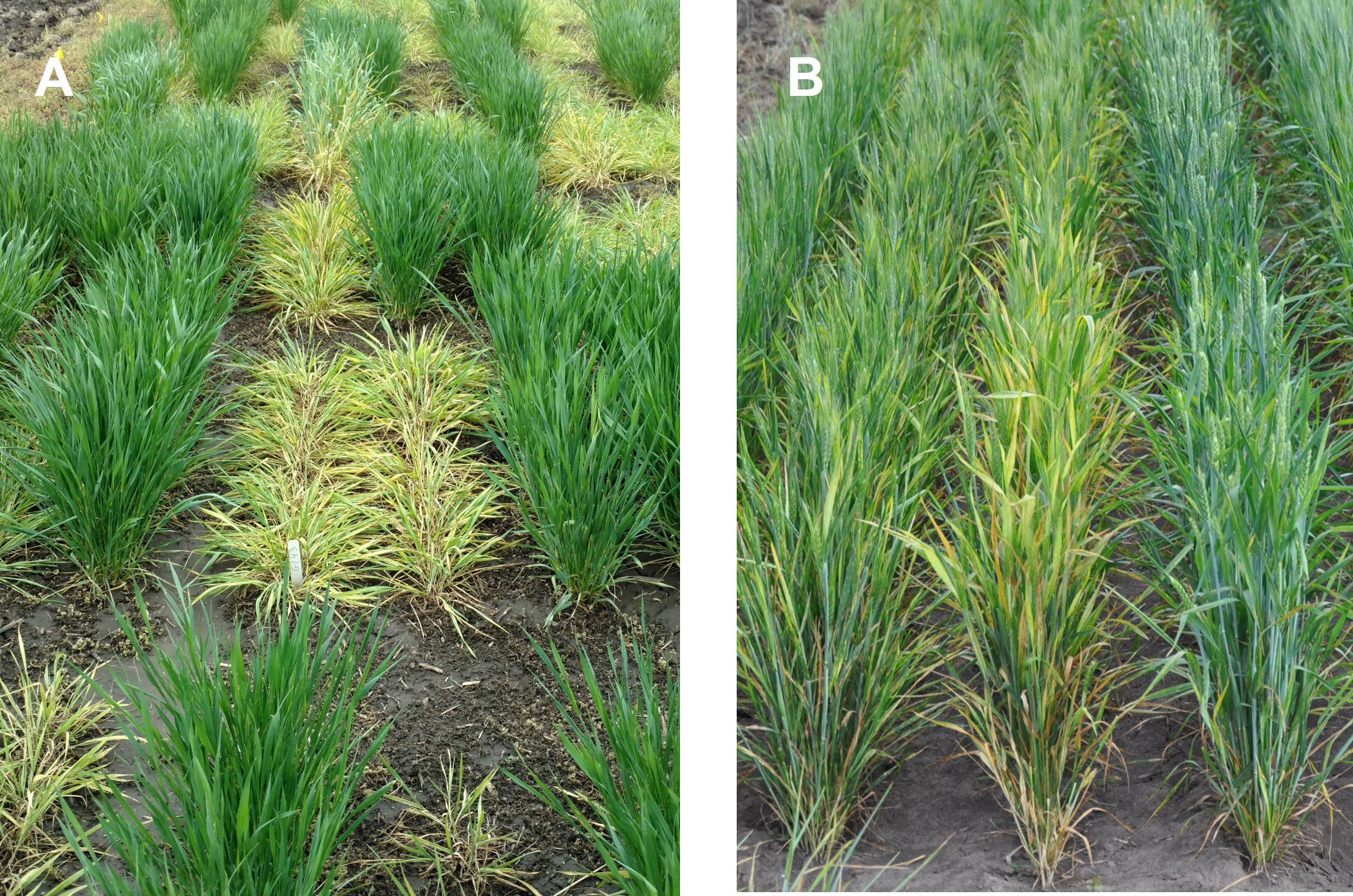
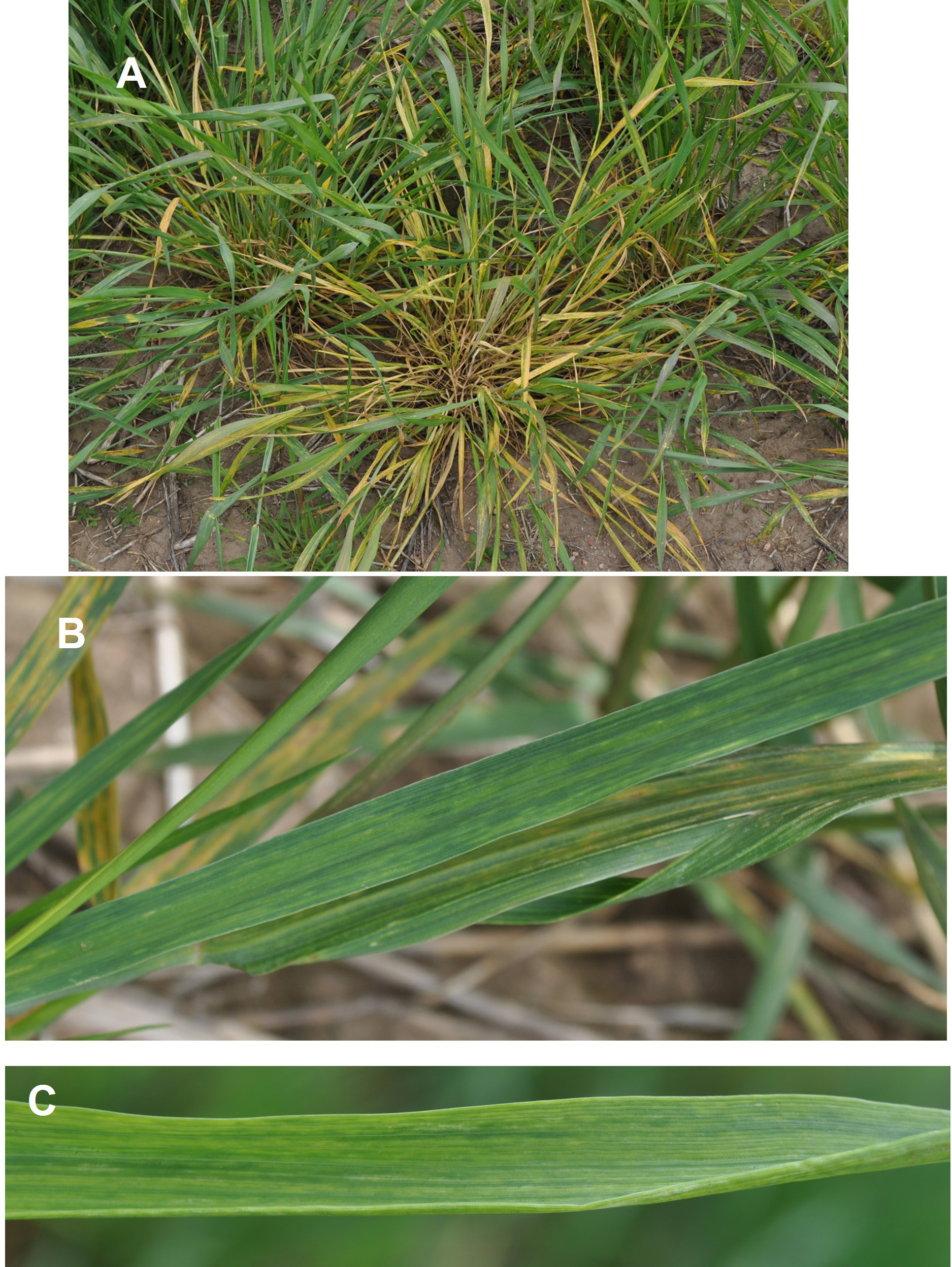
Fig. 5. Impact of mite transmitted viruses on susceptible winter wheat varieties (center of each photo) vs. virus-resistant varieties. A) Severity of early fall infection with severe stunting, prostrate tiller growth, and extreme yellowing. B) Late fall or early spring infection showing near normal upright growth, but leaf yellowing, slight stunting, reduced tiller number, and likely poor seed fill.
Fig. 6. Plants at field margins, neighboring a wheat curl mite source, are the first to become infected with viruses of the WSMV complex and develop symptoms, such as yellowing and streaking. Notice the gradient in color from the field edge (left) toward the center of the wheat field.
Plants in field margins closest to the source of wheat curl mites are usually the first and at times the only plants to show symptoms. At low to moderate levels of infection, there will likely be a gradation of the intensity of symptoms across a field (Figure 6). In severe epidemics, entire fields can show extreme symptoms, and complete yield loss is common (Figure 7).
Fig. 4. Whole plant and leaf symptoms from infection with the WSMV complex on wheat. A) Infected plant showing prostrate growth of tillers and severe yellowing of older leaves. B) Symptoms of infection on young leaves showing ‘mosaic’ patterns of yellowing. C) Later symptoms on a leaf with more extensive yellowing.
Fig. 7. Severe field-wide infection from WSMV complex viruses can lead to complete yield loss. The wheat tillers show severe stunting and distorted, prostrate growth. Many tillers have died prematurely.
In winter wheat, wheat curl mite infestations and virus infections that result in serious yield losses begin in the fall. In the Central and Northern Great Plains, however, symptoms usually do not appear until the following spring, except when there are prolonged warm temperatures late into the fall. With warmer temperatures through the fall and winter in the Southern Great Plains, infections are more likely to become noticeable earlier. The appearance of symptoms in the fall is an indication that a severe epidemic is occurring, and extreme impact is likely by the following spring.
In winter wheat, initial infections with viruses in the WSMV complex occur during the fall when viruliferous (able to transmit the viruses) wheat curl mites are spread by wind from virus-infected volunteer wheat and other cereal and grass hosts to the newly emerged wheat crop on which they feed and transmit the viruses (Figure 8).
Reproduction and spread of wheat curl mites stops in the fall when temperatures drop below 50°F (10°C), but the mites survive below-freezing winter temperatures. Unless plants are killed, mites are likely to survive the winter. The mites overwinter as eggs, larvae, nymphs, or adults in the host crown, and the viruses overwinter in the live tissues of the wheat plant and other hosts. In the spring, when temperatures warm, wheat curl mites become active and are spread by wind within and between fields where they transmit the viruses to healthy plants. However, the effects of spring infections on symptom development and yield of winter wheat are usually much milder than fall infection (Figure 9).
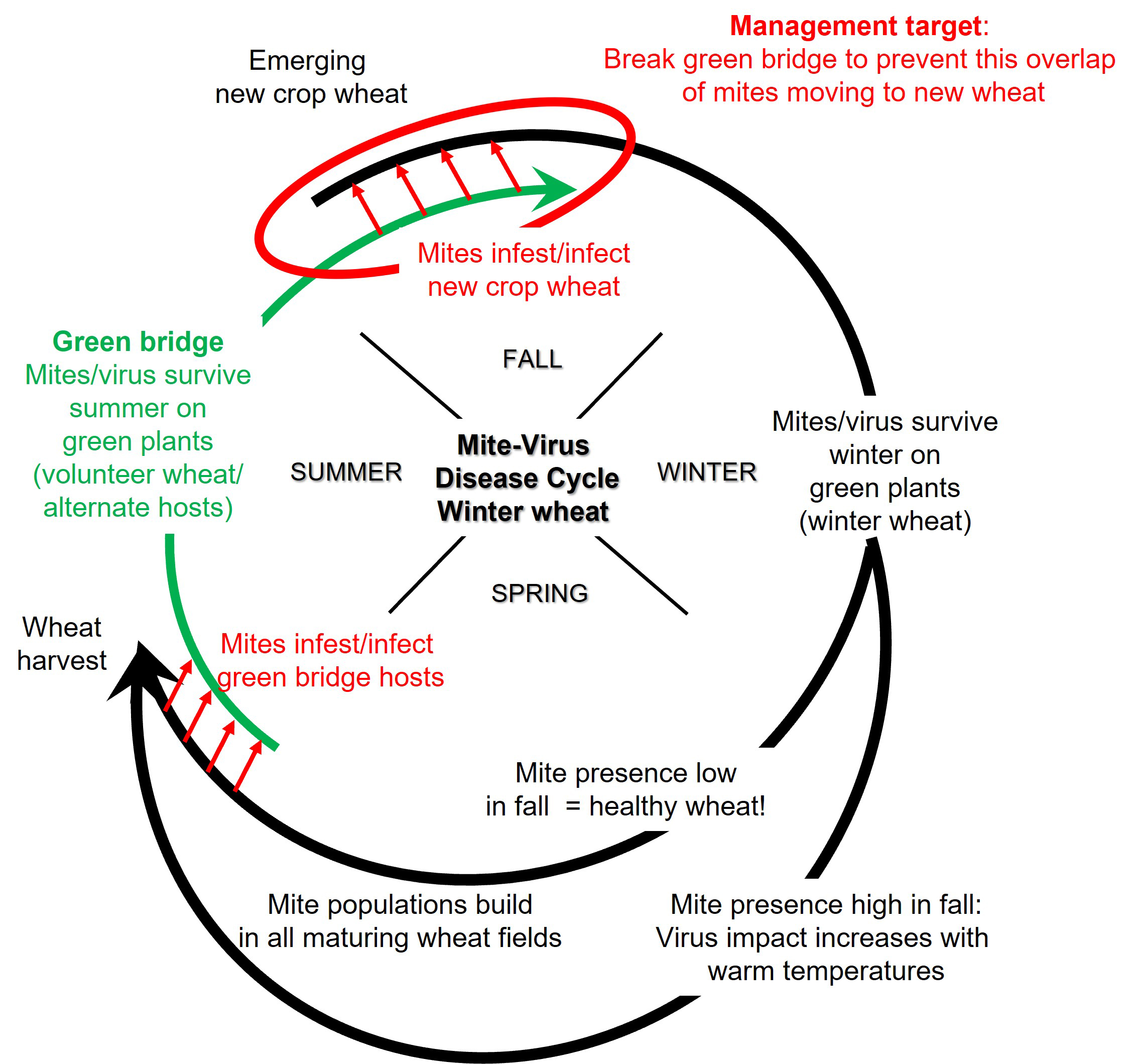
Fig. 8. Disease cycle for mite-transmitted viruses in winter wheat in the Great Plains.
As temperatures warm in the spring, symptoms become obvious and intensify in plants that were infected in the fall. During and after heading, wheat curl mites move to the heads (spikes) where they feed and are well protected. Mite populations build to high levels during head development. This occurs even in healthy wheat fields that showed no evidence of virus infection or wheat curl mite infestation during the current growing season. Therefore, when the wheat crop matures and starts to dry down, there are ample numbers of wheat curl mites that move off the wheat crop and must find new living hosts on which they can feed. These over-summering hosts serve as a ‘green bridge’ for the wheat curl mites and viruses to survive between wheat harvest and the emergence of the subsequent crop in the fall.

The highest-risk green bridge host is volunteer wheat that emerges before wheat harvest (pre-harvest volunteer wheat). A high abundance of pre-harvest volunteer wheat is often the result of hail events occurring during the late stages of wheat crop development. Wheat is most likely to produce pre-harvest volunteer when it is in the soft to hard dough stages, about 3 weeks before harvest. The wheat curl mites then move from the heads of the maturing wheat crop to the emerging volunteer wheat and transmit the viruses. Pre-harvest volunteer wheat will almost always become heavily infested with wheat curl mites and infected with viruses.
The preferred host for the wheat curl mite is wheat. However, several other cereal crops (e.g., corn, rye, oats, barley, sorghum, foxtail millet) and grasses (e.g., jointed goatgrass, cheatgrass [aka downy brome], sandbur, crabgrass, barnyardgrass, stinkgrass, witchgrass, green foxtail [aka green bristlegrass]) are hosts to the mite and viruses. Wet weather during summer facilitates lush growth of green bridge hosts, resulting in the buildup of high populations of mites that can transmit the viruses to the successive winter wheat crop. Following planting, the mites are spread by wind onto the newly emerged wheat crop and transmit the viruses, completing the disease cycle.
Spring wheat planted near wheat curl mite-infested winter wheat fields or fields with infested volunteer wheat is at high risk of virus infection. The disease cycle in spring wheat is similar to that in winter wheat with initial infections occurring in spring after wheat emergence. Additionally, the disease cycle can continue if mites are able to move from maturing spring wheat onto the newly emerging winter wheat in the fall.
Since the identification of the wheat curl mite as the vector of WSMV in the 1950s, proper identification of the mite has been problematic. A major reason for this difficulty has been the tiny size of the mite (about 0.01 in, or 0.25 mm). Recently, researchers around the world have identified several different genetic types of wheat curl mites and have found that they likely are a complex composed of subspecies or perhaps multiple species that are nearly identical in appearance. Thus far in North America, only two distinct genetic types of wheat curl mite (Type 1 and Type 2) have been identified. We do not know the full extent of the distribution of these two mite types, but it appears that both are widely distributed across the Great Plains. These two types have been found to differ in their ability to vector viruses of the WSMV complex. Both types will effectively transmit WSMV, but Type 2 wheat curl mites are much more effective at transmitting both HPWMoV and TriMV. The two types also differ in their reaction to wheat curl mite resistance genes in wheat, but it appears that even within the genetic types, the response to different resistance genes in wheat can be variable.
Fig. 9. Effect of fall and spring infection with WSMV on yield of six winter wheat varieties in Oklahoma. All yields of spring- and fall-infected winter wheat are significantly different from the uninoculated check, except for spring-infected Siouxland.
The wheat curl mite life cycle includes four stages (egg, two immature stages, and adult), and can be completed in about 7 to 10 days depending on temperature. After wheat curl mite eggs hatch, only the two immature stages (nymphs) can acquire WSMV by feeding on infected leaves. It can take as little as 15 minutes for the mite to acquire WSMV, and mites remain viruliferous for most of their lives (2–4 weeks or longer with cool temperatures), but the transmission efficiency of adult mites decreases with age. These transmission characteristics are similar for TriMV, but they remain unknown for HPWMoV.
Wheat curl mites do not have wings or produce webbing; thus, they depend almost entirely on the wind for dispersal. As wheat curl mite populations increase, mites leave the protected areas of their hosts (rolled or overlapped leaves, heads, etc.) to become airborne. After landing on a new host, the mites crawl to the youngest leaf and begin to feed and reproduce. A single mite carrying the virus is enough to infect an entire plant.
Wheat curl mite densities reach their highest level in the entire year at the time wheat approaches maturity. The mites find numerous secluded sites for feeding and protection within the wheat heads, and their populations build to very high levels. Even in fields that have shown no evidence of virus infection through the year, densities can reach 500 to 1,000 mites per head. This is equivalent to about 1 to 2 billion mites per acre of wheat. As wheat matures and dries down, these mites begin to move off the plants and rain down throughout the surrounding ecosystem; this is called the ‘mite-rain period.’ In areas where wheat is widely grown, wheat curl mites will infest every potential host in the surrounding landscape at this time, but they will survive and reproduce only on those hosts that are suitable. After wheat harvest, wheat curl mite activity drops to very low levels until mite population densities build up in the green bridge hosts.
The major factor in WSM disease outbreaks in winter wheat is the presence of over-summering hosts that enable wheat curl mites to carry the viruses from the previous wheat crop, build up to large numbers during the summer, disperse in the fall, and transmit the viruses to the newly emerging wheat crop. Wheat curl mites are unable to survive for more than a day or two off green plants. Therefore, they must disperse to find a living green bridge host to survive through the summer. Across the Great Plains, green bridge host relationships change depending on green bridge length (harvest to fall emergence period) and host availability, density, and quality.
The most important green bridge host across the Great Plains is volunteer winter wheat that emerges before wheat harvest. Pre-harvest volunteer wheat is most often a result of hailstorms. When hail shatters grains from developing heads during the last three weeks before harvest (soft to hard dough stages) and adequate moisture is present, kernels will readily sprout and produce volunteer seedlings. Wheat varieties can vary in their susceptibility to this rapid germination and resulting volunteer emergence. Wheat curl mites readily infest this volunteer wheat on which mites and viruses multiply rapidly. If pre-harvest volunteer wheat survives through the green bridge period, the extensive, viruliferous mite populations that develop will move onto emerged winter wheat plants and transmit the viruses (Figure 10).
During the mite-rain period just before wheat harvest, any growing host near wheat fields will become infested with wheat curl mites, including cereal cover crops, corn, foxtail millet, and volunteer wheat growing within summer crops (e.g., corn, sunflower, millet), as well as other alternate grass hosts. However, if volunteer wheat or these other hosts do not emerge until after wheat harvest (post-harvest volunteer wheat), the potential for serious mite infestations is much reduced. The disease risk through the green bridge period is also tied to precipitation prior to planting the new crop. Late-season rains allow the volunteer wheat and other potential green bridge hosts to grow well and better serve as a source of the viruses and mites. Some mite activity may persist at low levels after harvest if mites are found on hosts in the surrounding landscape, and they may spread to infest either post-harvest volunteer wheat or early planted winter wheat.
The role of post-harvest volunteer wheat as a significant wheat curl mite source is dependent on the length of time it has been growing during the green bridge period and the presence of important mite hosts in surrounding areas (e.g., spring wheat, pre-harvest volunteer wheat, corn, and other hosts). For example, in western Nebraska, post-harvest volunteer wheat has a relatively low risk of severe mite populations developing unless a significant mite source (pre-harvest volunteer wheat) is nearby to provide initial mite infestation. However, in areas to the south (Kansas, Oklahoma, and Texas) harvest occurs earlier and winter wheat is typically planted later, unless it is used for grazing (this is discussed in the section below titled ‘Regional Differences in Disease Occurrence and Impact’). This allows more time for small wheat curl mite populations to infest post-harvest volunteer wheat and build up to significant densities for dispersal into the wheat crop later in the fall.

Fig. 10. Wheat curl mite movement and virus spread, resulting in serious WSM risk, originate from green bridge hosts, especially pre-harvest volunteer wheat. A, B) Volunteer originating from pre-harvest hail in Nebraska. C) Post-harvest volunteer (right) growing through the long green bridge period in Texas serving as a mite source for new crop wheat (left), and D) Volunteer wheat growing at wheat harvest will become heavily infested with wheat curl mites, such as this volunteer wheat seen after sunflower harvest.
Both wheat curl mites and viruses of the WSMV complex are dependent on a green living host for their survival. There are two major periods that threaten mite populations. The first is to survive the winter. Winter wheat maintains living tissue through the winter and provides an ideal host for the mite to survive well through the winter. The second major challenge for the mites is surviving through the summer between wheat harvest and emergence of the winter wheat crop in the fall. Therefore, green bridge hosts are extremely important to the epidemiology of WSM disease.
Wheat curl mites and WSMV can survive on barley, oats, corn, rye, and numerous grassy weeds, but wheat is their ideal host. Neither wheat curl mites nor any of the viruses can survive on any broadleaf plants. More than 90 grass species have been identified as potential mite hosts, and a number of these have been shown to host one or more of the viruses in the WSMV complex. However, data on the quality of these grass species as potential hosts are highly variable.

There are four major requirements that must be met for grasses to pose a significant risk as green bridge hosts for the development of significant levels of WSM:
Some of the best wheat curl mite and virus hosts that meet requirements 1 and 2, such as jointed goatgrass, do not serve as good green bridge hosts, because they match the seasonality of winter wheat and do not survive through the green bridge period (requirement 4). Other hosts are not commonly found in great enough densities in the surrounding environment (requirement 3) to support wheat curl mite populations at densities necessary to pose a significant risk. Barnyardgrass is a very good host for the mite, and it can host WSMV, as well (Figure 11A). In the field, however, it grows in patches, and densities of this grass host are seldom extensive across entire fields. This limits its risk as a green bridge host. Green foxtail (Figure 11B) can host wheat curl mites, but it is not nearly as good a host as barnyardgrass. However, it is often much more prevalent than barnyardgrass, so it may still have some risk as a green bridge host.
Fig. 11. Alternate grass hosts for wheat curl mites and viruses in the WSMV complex. A) Barnyardgrass (Echinochloa crus-galli), B) Green foxtail (aka green bristlegrass; Setaria viridis), C) Cheatgrass (aka downy brome; Bromus tectorum). Figs. 11A and 11B. Howard F. Schwartz, Colorado State University, Bugwood.org.Fig.11C. Chris Evans, University of Illinois, Bugwood.org.
Another example to illustrate the value of evaluating the four host requirements is cheatgrass (aka downy brome; Figure 11C), an invasive species that is very common in the Central and Southern Great Plains. This winter annual is a reasonably good host for wheat curl mites and at least WSMV (requirements 1 and 2), but in these two regions of the Great Plains it matures, dries down, and dies in the summer, well before the wheat crop, and seeds do not germinate until early fall (requirements 3 and 4). Thus, it is a very poor green bridge host in these areas. However, seasonal growing conditions in the Northern Great Plains result in cheatgrass growing through the summer into the early fall and overlapping with the emergence of fall-planted winter wheat. Thus, in the Northern Great Plains, cheatgrass may pose a greater risk as a green bridge host, because it fulfills all four requirements.
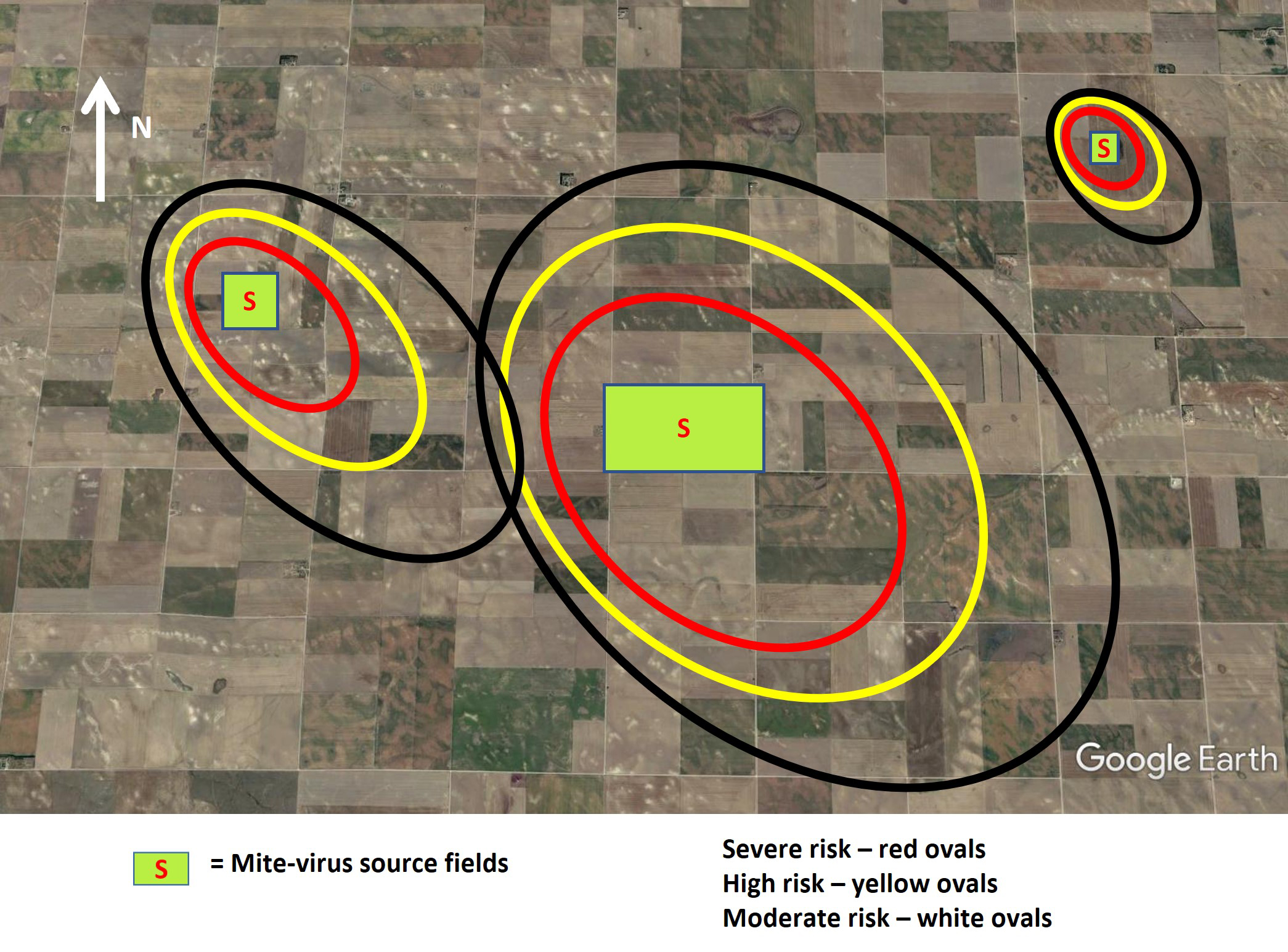
Fig. 12. The ‘sphere of influence’ for wheat curl mite movement and virus spread extends in all directions in an oval pattern surrounding the mite source with the long axis of the oval in the direction of prevailing winds (e.g., northwest in the central Great Plains). The risk of virus infection is highest close to the source field and decreases with increasing distance from the source field. It can extend out 1 to 2 miles.
The risk of wheat curl mite dispersal and virus spread from a source field, such as a pre-harvest volunteer wheat field, extends in all directions from the source, but it tends to follow an oval-shaped pattern extending farther away from the direction of the prevailing winds (Figure 12). In the Central Great Plains, this sphere of influence around the source most often extends to the southeast away from the high prevailing northwest winds that regularly occur in the fall. The extent of wheat curl mite spread is dependent on the size (area), green bridge host density, and mite density within the green bridge host.
If wheat curl mite populations in the source field are low, spread to neighboring fields will only occur for a short distance, and an edge effect of the spread will be evident (Figure 13). If mite populations in the source field are high and the source field is large, wheat curl mites and viruses will spread across entire fields neighboring the source field and perhaps well beyond (Figure 13). The distance of significant spread may extend up to 1 to 2 miles. The risk of serious virus spread will decrease with distance from the source field, but the extensive sphere of influence surrounding significant mite source fields necessitates optimal management to protect both adjoining fields and those in the surrounding neighborhood.
The increased risk for wheat curl mite spread and virus infection throughout a neighborhood landscape is particularly evident when large hail streaks result in hail losses, and subsequent pre-harvest volunteer wheat is prevalent. When there are numerous source fields present and their sphere of influence is overlapping, the risk of wheat curl mite and virus spread across these and surrounding areas is elevated and may extend well beyond those areas directly impacted by hail. These situations especially require a concerted neighborhood-wide effort in controlling pre-harvest volunteer wheat to avoid serious WSM disease risk to the subsequent wheat crop.
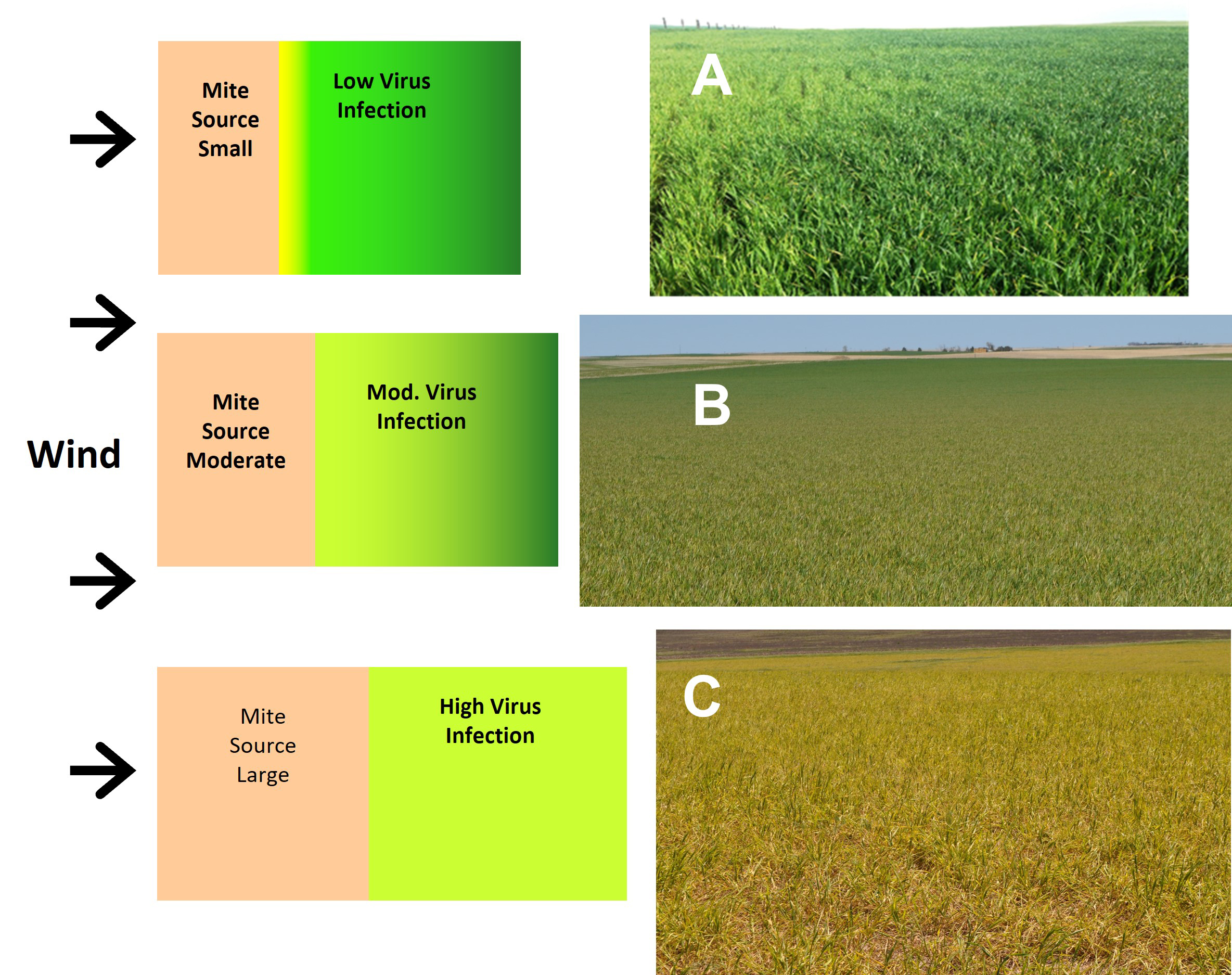
Fig. 13. Wheat curl mite movement and virus spread are governed by the size of the mite source. The size of the mite source is determined by the area of the source field and the density of the host plants and mite population present in that source field. As the mite source increases in size, virus spread increases from border effects (left to right in A) to an extensive gradient (front to back in B) to whole field destruction (C).
Wheat curl mite movement and virus spread occur in the new wheat crop throughout the fall as mites continue to disperse from unmanaged source fields when conditions are favorable. Early winter wheat planting and long periods of mild weather extending through October and November increase the risk of wheat curl mite and virus spread and disease development. Under very warm fall conditions, the probability of secondary spread of mites and viruses also increases, resulting in greater incidence of infection. Secondary spread results from mite populations increasing in densities after initial infestation of the new wheat crop and a portion of those mites spreading farther into the new wheat crop.
Wheat production practices and growing conditions vary across the U.S. Great Plains, and these, in turn, influence the occurrence and severity of mite-transmitted viruses. In the Southern Great Plains, especially in Oklahoma and Texas, winter wheat is commonly planted for two purposes: fall grazing and for grain the following spring. Early planting of winter wheat in late August through late September allows for sufficient plant growth to graze cattle in late fall through the winter. This early planting, however, means a shorter time to break the green bridge over the summer, and it also increases the risk for mite infestation and virus infection. In irrigated fields, the four corners of the center-pivot circle are usually planted with wheat when the pivot-irrigated field is planted with corn or cotton. The wheat in the four corners can become infected with WSMV and serve as volunteer wheat when the field is back into rotation with wheat. Moreover, the warmer fall and milder winter conditions in the Southern Great Plains promote survival and continued buildup of wheat curl mites on winter wheat. As a result, the Southern Great Plains tends to have frequent occurrence of mite-transmitted viruses in winter wheat (Figure 14).
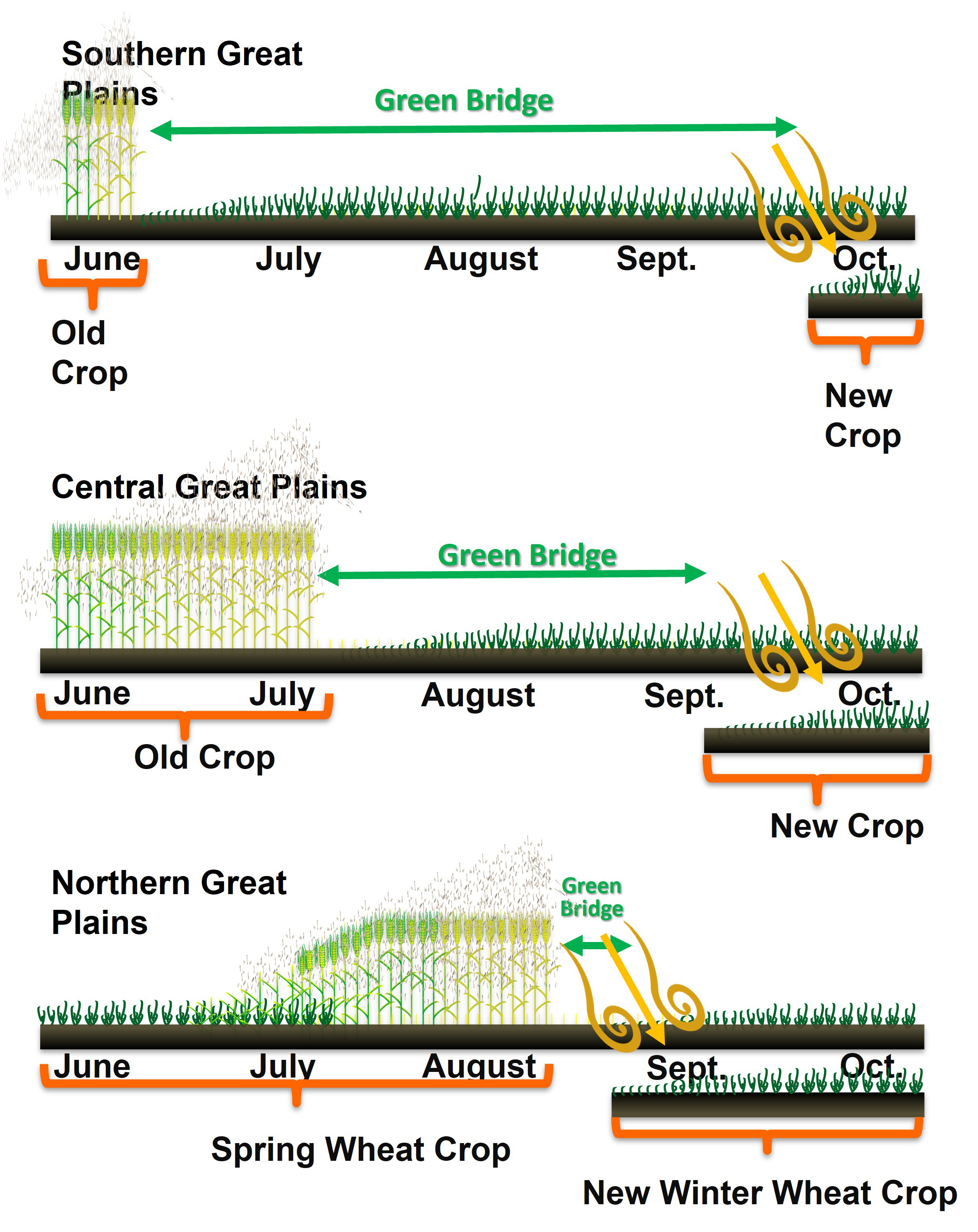
Fig. 14. The seasonal importance of the green bridge changes across the Great Plains with the green bridge becoming shorter from south to north. These changes alter the risk factors for virus impact and eventual management of this disease complex.
In the Northern Great Plains, wheat is typically grown in wheat–fallow rotation or followed with crops that are not hosts of wheat curl mites and viruses in the WSMV complex, such as pulse crops. The practice of fallowing fields to conserve soil moisture encourages growth of volunteer wheat and grassy hosts such as cheatgrass, both of which are suitable hosts for mites and the viruses. In addition, the Northern Great Plains can have high acreage of spring cereals including spring wheat, durum, and barley, which are susceptible to wheat curl mites and viruses in the WSMV complex. Spring cereals mature later than winter wheat, and can serve as over-summering hosts for mites dispersing from maturing winter wheat. If winter wheat is being planted early and emerges during or shortly after spring cereal harvest, it will be at greater risk for mite infestation and virus infection. Extended warm falls with normal or above-normal moisture further increase disease risk by prolonging the period during which summer and winter crops co-occur. Above-normal precipitation amounts will also hinder volunteer and grassy weed control, resulting in inadequate management of green bridge hosts that harbor mites and viruses.
Management of wheat curl mite-transmitted viruses is difficult. It has historically relied on cultural practices to eliminate over-summering hosts of the mite, because no chemicals or highly resistant varieties were available. Chemical control of the wheat curl mite remains challenging, because of the mite’s reclusive nature of feeding in the whorl and other recesses of the plant. However, wheat varieties with varying levels of resistance to viruses in the WSMV complex and the wheat curl mite are becoming more available. Hence, options are increasing to help achieve management of the disease. Another difficult aspect related to managing the mite-virus complex in wheat is the responsibility of being “a good neighbor” to help limit spread of the disease to neighboring wheat fields. On occasion, lawsuits have been threatened or filed in court to attempt to recover losses from WSM when one producer felt a neighbor did not take adequate steps to limit spread of the disease by destroying the green bridge in their field. A communal effort to break the green bridge involving growers, crop consultants, and Extension personnel is an effective approach to reducing the risk of WSMV outbreaks and the associated yield losses.
Management of this wheat-mite-virus complex seeks to minimize or eliminate the risk of infection. Wheat curl mite presence overrides all risk factors. If mites are not present, other risk factors will not impact the level of risk. Thus, many of the primary risk factors deal with managing green bridge hosts for wheat curl mites.
Factors increasing risk of virus infection (highest risks listed first):
Environmental factors that increase virus risk:
Wheat streak mosaic management becomes more important the higher the risk of disease, but risk factors can be variable between states, counties, and even fields As a result, this makes it challenging to assess risks and develop management strategies in response. ‘AWaRe’ (Assessment of Wheat streak mosaic Risk) is an online learning tool designed to assist users in identifying and evaluating factors contributing to WSM risk with a focus on disease dynamics in the Northern Great Plains. It transparently dissects the most important factors relating to WSM risk through five applied questions that assess WSM virus presence, wheat curl mite dispersal success, and survival of the mites and viruses between crops. AWaRe has an interactive design that invites users to explore the composition of WSM risk in the Northern Great Plains and makes this application an alternative learning resource on the WSM disease complex. This learning tool can be used to assess conditions in a specific wheat field of interest, or to dissect WSM disease risk in hypothetical scenarios. The AWaRe tool is designed to allow the assessment of WSM risk before a host crop is planted so that producers can implement appropriate management strategies to help prevent WSM infection. AWaRe focuses on WSM risk factors specific to Montana and the Northern Great Plains. AWaRe can be accessed through the Montana State University Extension Plant Pathology website: http://plantpath.msuextension.org/resources/#plant-path-tools.
One of the primary cultural practices to mitigate WSM disease is the elimination of volunteer wheat and other green bridge hosts for a period of at least two weeks before the new winter wheat crop emerges. This practice is commonly referred to as ‘breaking the green bridge.’ A two-week host-free period helps eliminate the reservoir of wheat curl mites that otherwise would transmit the viruses to emerging wheat. Volunteer wheat and grass weeds can be destroyed by tillage or by herbicide application, but it is critical that volunteer wheat and grass weeds are completely dead for a period of at least two weeks prior to crop emergence.
A second beneficial practice to manage WSM disease is to avoid early planting of winter wheat. Later planting dates will allow less time for wheat curl mites to infest the wheat in the fall and provide more time to control the volunteer wheat and grass hosts. Wheat infected in the fall is damaged much more severely than when virus infection occurs in the spring. Where winter and spring wheat crops are grown in the same area, early planting of spring wheat should allow the crop to become established before the risk of WSM disease spread increases with warm temperatures and declining conditions of the mite source, which result in increased dispersal of the mites.
The type of cropping system is another factor that can influence management of wheat curl mite-transmitted virus diseases. For example, management in reduced- or no-till systems can be more difficult compared to management in a traditional tillage system, because volunteer wheat can emerge after each rainfall event rather than in a large flush after a rainfall event as in a traditional tillage system. Thus, breaking the green bridge in a no-till system may require multiple herbicide applications in years with high precipitation.
Risk can be reduced by diversifying crop rotation in no-till wheat production systems by incorporating crops such as pulses that do not host wheat curl mites and the associated viruses. This reduces the concentration of wheat and the size of the area in the landscape where volunteer wheat may be problematic through the summer.
Historically, an occasional wheat variety was released (e.g., ‘Rall’ in the 1970s) that exhibited a low level of resistance to WSM. However, these early resistant varieties were either not agronomically competitive or the resistance was not highly effective. Better resistance was later found in wheat and wheat relatives, and involved genes that conferred resistance either to WSMV or the wheat curl mite. Transferring the genes conferring this resistance into adapted bread wheat varieties has been slow, but recently there have been several winter wheat varieties released with resistance to either the virus or to the wheat curl mite.

Fig. 15. WSMV screen showing the impact of the virus complex on various varieties with WSMV resistance genes (Wsm) or wheat curl mite resistance genes (Cmc). The remaining entries are susceptible commercial varieties.
To date, two virus resistance genes have been used to develop commercial winter wheat varieties. The gene Wsm1 showed strong levels of resistance to both WSMV and TriMV, but its incorporation into wheat lines resulted in enough yield drag that the variety was not widely grown (e.g., ‘Mace’) (Figure 15). A second gene, Wsm2, has been more widely incorporated into commercially released lines (e.g., ‘Ron L’, ‘Snowmass’, ‘Clara CL’, ‘Oakley CL’); however, these lines only provide resistance to WSMV and perform poorly where TriMV is prevalent (Figure 15). Additional genes (Wsm3 and Wsm4 to date) are being evaluated, and these may provide more consistent resistance in the near future.
There are several challenges with currently available virus-resistant varieties. First, there are three wheat curl mite-transmitted viruses, but the genetic resistance in these varieties may provide resistance to only a single virus, as with Wsm2. Second, the virus-resistance in both Wsm1 and Wsm2 is temperature sensitive, and begins to break down as temperatures rise above about 70°F (ca. 20°C). Thus, these varieties limit fall and early spring virus establishment, and they are likely to be more effective where fall temperatures are cooler. However, earlier planting under warmer conditions will limit their effectiveness, because wheat with this temperature-sensitive resistance does not recover from the early season infection.
Resistance to wheat curl mites has been pursued for some time. In the 1970s and 1980s, a mite resistant gene from rye was incorporated into wheat and released in the commercial wheat line ‘TAM 107’. This line became very popular in some areas of the Great Plains, but after several years of widespread use, wheat curl mite populations began to survive much better on this variety, thus eliminating its value for mite resistance. There are other wheat curl mite resistance genes that have been and are being incorporated into commercial lines, but the stability of these to avoid mites overcoming the resistance is unknown. However, field trials have shown some value to this type of resistance (Figure 15). Perhaps the most important advantage of mite resistance will be seen in the reduction of wheat curl mite population growth in volunteer wheat, thus limiting mite buildup during the green bridge.
An often-posed question is: what should producers do if a wheat field exhibits symptoms of WSM disease? Once it is confirmed that one or more wheat curl mite-transmitted viruses are causing the symptoms, an assessment needs to be made of how widespread the disease is in the field and if yield will be significantly impacted. This will lead to the decision to continue the field to harvest, baling the wheat for hay, “grazing out” the field with cattle, or abandoning the field. Typically, such a decision is based on an assessment of the yield potential of the field made by a crop insurance adjuster and the timing relative to the insurance release date. Consideration of what would best fit with the individual producer’s cropping plan should be factored into the decision-making process.
Additional inputs in fields where WSM disease is widespread should be avoided to minimize financial loss. Infected plants will not recover, and they will continue to deteriorate under warm conditions. Studies have found that adding nitrogen to wheat plants promotes wheat curl mite population growth and increases the plants’ susceptibility to WSMV infection. Moreover, WSMV infection of wheat reduces root development and grain and forage yields, resulting in reduced water use efficiency. For irrigated wheat, this means that once the crop shows symptoms of WSM disease, increases in water applications are unlikely to increase crop productivity. If possible, irrigation practices should be adjusted site-specifically in accordance with the patterns of disease in the field (i.e., reduce water applications in WSMV-affected areas of the field) to conserve resources.
Grazing out wheat fields may be a feasible strategy to manage a WSMV-affected crop, and this does not appear to increase the risk of spreading the disease. Experiments designed to investigate the effect of sheep grazing of WSMV-infected wheat on disease risk did not produce any evidence that the virus is spread from infected to healthy wheat plants by grazing sheep (salivary transmission). The grazing did not increase wheat curl mite population sizes in the infested crop either, likely because plant biomass that provides shelter and feeding sites to the mites was reduced by grazing.
Chemical termination of virus-affected fields (or green bridge hosts) is commonly done with glyphosate herbicide. However, the timing of crop termination needs to be carefully considered to avoid wheat curl mite and virus spread to adjacent crops. Glyphosate-treated wheat plants can be a source of dispersing wheat curl mites until the plants have died back to the crown. This can take up to two weeks following the application. Short-term increases in wheat curl mite population size and dispersal have been observed in the three to nine days following glyphosate treatment, depending on the glyphosate dosage applied. This suggests that the risk of wheat curl mite dispersal can increase in the first days following a glyphosate application. Host plant termination using paraquat herbicide appears to act more quickly, and wheat curl mite populations on treated plants decline within a few days. If it is an option, tillage will reduce mite populations within a few days, as well, as long as plants are destroyed completely.
Being a “good neighbor” is an important component to help limit the occurrence and spread of WSM disease to adjacent fields of wheat. Individual growers may be doing all they can to reduce the risk of infection within their field, but if the green bridge in adjacent fields is not controlled, these management efforts will have little effect. In the past, there have been attempts to litigate losses seen in commercial fields adjacent to fields where volunteer wheat was not controlled. In one such case, the courts ruled that “. . . there is no common-law duty in Kansas for landowners to control volunteer wheat for the purpose of preventing outbreaks of wheat streak mosaic” (Kan.App.,1997. Krug v. Koriel; 23 Kan.App.2d 751, 935 P.2d 1063). This indicates that managing wheat curl mite-transmitted virus diseases is difficult and will be most successful if neighbors work in concert with each other.
Management of wheat curl mite-transmitted virus diseases is critical, because of the devastating effects the diseases can have on wheat production. Infections that occur in the fall are much more damaging than spring infections, with both forage production and grain yield being significantly impacted. Using cultural practices to break the green bridge and avoiding early planting of winter wheat currently provide the most promise for successful management of WSM disease. These practices, especially if coupled with planting resistant varieties, will help to significantly limit losses from wheat curl mite-transmitted virus diseases.
Almas, L.K., Price, J.A., Workneh, F., and Rush, C.M., 2016, Quantifying economic Losses Associated with Levels of Wheat Streak Mosaic Incidence and Severity in the Texas High Plains. Crop Protection 88:155–160.
Brey, C.W., Johnson, G.D., and Blodgett, S.L., 1998, Survey of Montana grasses for wheat curl mite (Acari: Eriophyidae), the vector of Wheat streak mosaic virus. Journal of Agricultural. Entomology 15:173–181.
Fahim, M., Dove, H., Kelman, W.M., Ayala-Navarrete, L. and Larkin, P.J., 2010, Does grazing of infected wheat by sheep result in salivary transmission of Wheat streak mosaic virus? Crop and Pasture Science 61:247–254.
Harvey, T.L., Martin, T.J., and Seifers, D.L., 1990, Wheat curl mite and wheat streak mosaic in moderate trichome density wheat cultivars. Crop Science 30:534–536.
Hein, G., 2019, Pre-harvest hail across western Nebraska necessitates breaking the green bridge: University of Nebraska–Lincoln CropWatch: at https://cropwatch.unl.edu/2019/pre-harvest-hail-necessitates-breaking-green-bridge (accessed February 2023).
Hollandbeck, G.F., DeWolf, E., and Todd, T., 2017, Preliminary 2017 Kansas wheat disease loss estimates: Kansas cooperative plant disease survey report, 6 p.
Hunger, R.M., Sherwood, J.L., Evans, C.K., and Montana, J.R., 1992, Effects of planting dates and inoculation date on severity of wheat streak mosaic in hard red winter wheat cultivars. Plant Disease 76:1056–1060.
Ito, D., Miller, Z., Menalled, F., Moffet, M., and Burrows, M., 2011, Relative susceptibility among alternative hosts prevalent in the Great Plains to Wheat streak mosaic virus. Plant Disease 96:1185–1192.
Miller, Z., Lehnhoff, E., Menalled, F., and Burrows, M., 2015, Effects of soil nitrogen and atmospheric carbon dioxide on Wheat streak mosaic virus and its vector, the wheat curl mite (Aceria tosichella Keifer). Plant Disease 99:1803–1807.
Murgan, M., Sotelo Cardona, P., Duraimurugan, P., Whitfield, A.E., Schneweis, D., Starkey, S., and Smith, C.M., 2011, Wheat curl mite resistance: Interactions of mite feeding with Wheat streak mosaic virus infection. Journal of Economic Entomology 104:1406–1414.
Oliveria-Hoffman, C., Wegulo, S.N., Tatineni, S., Hein, G.L., 2015, Impact of Wheat streak mosaic virus and Triticum mosaic virus coinfection of wheat on transmission rates by wheat curl mites. Plant Disease 99:1170–1174.
Price, J.A., Simmons, A.R., Rased, A., Workneh, F., and Rush, C.M., 2014, Winter wheat cultivars with temperature-sensitive resistance to Wheat streak mosaic virus do not recover from early-season infections. Plant Disease 98:525–531.
Skoracka, A., Rector, B.G., and Hein, G.L., 2018, The Interface Between Wheat and the Wheat Curl Mite, Aceria tosichella, the Primary Vector of Globally Important Diseases. Frontiers in Plant Science 9:1098. https://doi.org/10.3389/fpls.2018.01098.
Slykhuis, J.T., 1953, Wheat streak mosaic in Alberta and factors related to its spread. Canadian Journal of Agricultural Science 33:195–197.
Slykhuis, J.T., 1955, Aceria tulipae Keifer (Acarina: Eriophyidae) in relation to the spread of wheat streak mosaic. Phytopathology 45:116–128.
Somsen, H.W. and Sill, W.H., 1970, The wheat curl mite, Aceria tulipae Keifer, in relation to epidemiology and control of wheat streak mosaic. Research Publication 162, Kansas Agricultural Experiment Station.
Staples, R., and Allington, W.B., 1956, Streak mosaic of wheat in Nebraska and its control: University of Nebraska College of Agriculture Agricultural Experiment Station. 41 p.
Stilwell, A.R., Rundquist, D.C., Marx, D.B., and Hein, G.L., 2019, Differential spatial gradients of Wheat streak mosaic virus into winter wheat from a central mite-virus source. Plant Disease 103:338–344.
Tatineni, S. and Hein, G.L., 2018, Genetics and mechanisms underlying transmission of Wheat streak mosaic virus by the wheat curl mite. Current Opinion in Virology 33:37–54.
Tatineni, S., and Hein, G.L., 2021, High Plains wheat mosaic virus: An enigmatic disease of wheat and corn causing the High Plains disease. Molecular Plant Pathology 22:1167–1179.
Velandia, M., Rejesus, R.M., Jones, D.C., Price, J.A., Workneh, F., and Rush, C.M., 2010, Economic Impact of Wheat Streak Mosaic Virus in the Texas High Plains. Crop Protection 29:699–703.
Wegulo, S.N., Hein, G.L., Klein, R.N., and French, R.C., 2008, Managing wheat streak mosaic, University of Nebraska–Lincoln Extension Circular EC1871, 8 p.
Workneh, F., Jones, D.C., and Rush, C.M., 2009, Quantifying Wheat Yield across the Field as a Function of Wheat Streak Mosaic Intensity: A State Space Approach. Phytopathology 99:432–440.
Workneh, F., Price, J.A., Jones, D.C., and Rush, C.M., 2010, Wheat streak mosaic: A classic case of plant disease impact on soil water content and crop water-use efficiency. Plant Disease 94:771–774.
Wosula, E.N., McMechan, A.J., Oliveira-Hoffman, C., Wegulo, S.N., and Hein, G.L., 2016, Differential transmission of two isolates of Wheat streak mosaic virus by five wheat curl mite populations. Plant Disease 100:154–158.
This publication has been peer reviewed.
Nebraska Extension publications are available online at http://extensionpubs.unl.edu/.
Extension is a Division of the Institute of Agriculture and Natural Resources at the University of Nebraska–Lincoln cooperating with the Counties and the United States Department of Agriculture.
Nebraska Extension educational programs abide with the nondiscrimination policies of the University of Nebraska–Lincoln and the United States Department of Agriculture.
© 2023, The Board of Regents of the University of Nebraska on behalf of the Nebraska Extension. All rights reserved.