G1678
The Relationship of Dry Bean and Sugar Beet Pathogens with Common Weeds in Nebraska Production Fields
Certain weeds in the Nebraska Panhandle can serve as hosts for pathogens that cause disease in dry edible beans and sugar beets. Properly managing weed populations and rotating with nonhost crops can help control the spread of disease.
Robert M. Harveson, Extension Plant Pathologist
Introduction
Dry edible beans and sugar beets are two very important crops to the farm economy in western Nebraska. Traditionally they have been grown in rotation with several other crops including corn, wheat, and alfalfa. One of the primary reasons that growers rotate additional crops with beans and beets is to attempt to reduce the likelihood of experiencing severe disease problems.
Both weeds and crop volunteers the following season can potentially harbor pathogens that could be detrimental to economically important field crops. This publication is meant to alert growers and consultants to the potential hazards that certain weeds in the Nebraska Panhandle (and surrounding central High Plains — Wyoming and Colorado) can pose as hosts for pathogens, and how to recognize disease symptoms on both weeds and crops.
Survey Background
Surveys have been conducted periodically over the last decade with the purpose of identifying weeds that exhibit symptoms of disease (root rotting and/or leaf spots and blights). The pathogens that were considered for this study include Cercospora beticola, Aphanomyces cochlioides, Rhizoctonia solani, Pythium spp., Fusarium oxysporum, and Polymyxa betae/beet necrotic yellow vein virus (BNYVV), causal agents of Cercospora leaf spot, Aphanomyces root rot, Rhizoctonia root rot, Pythium root rot, Fusarium wilt, and rhizomania, respectively. Also included in this survey was the false root knot nematode (Naccobus aberrans) and two newly identified diseases of lambsquarters that could be confused with similar diseases of sugar beets (Ramularia leaf spot and downy mildew).
Three primary weeds common to western Nebraska were also considered, including redroot pigweed (Amaranthus retroflexus), common lambsquarters (Chenopodium album), and fireweed (Kochia scopularia). All three of the weeds included in this study have been reported in the literature as hosts for one or more of the sugar beet or dry bean pathogens. Based on these literature sources, redroot pigweed appears to be the host capable of harboring the greatest number of pathogens, including F. oxysporum, R. solani, A. cochlioides, Cercospora, Pythium, and Polymyxa betae. Lambsquarters has been reported to be infected by Cercospora, R. solani, A. cochlioides, P. betae, and N. aberrans, while Kochia (fireweed) is a host for R. solani, Cercospora, Pythium, P. betae, and N. aberrans. All of these pathogens additionally cause disease in sugar beets, which is not surprising since lambsquarters and Kochia are in the Chenopodiaceae family with sugar beets, and pigweed is in the closely related Amaranthaceae family.
The list of pathogens reported on weeds that also affect dry beans is much shorter than that from sugar beets. These pathogens include R. solani and Pythium spp., which is important to note because both of these pathogens can also infect sugar beets. Even if weeds were adequately controlled, close rotations with both beets and beans could still potentially increase disease problems. The Fusarium and Aphanomyces pathogens that infect sugar beets will not damage dry beans, and vice versa. However, it has been shown that all four pathogens found on weeds are also pathogenic on sugar beets. Conversely, the Ramularia leaf spot and downy mildew disease on lambsquarters are not pathogenic on sugar beets.
Results
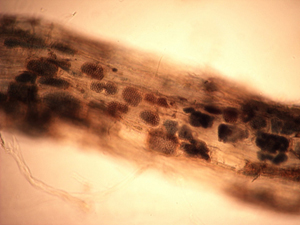
Working with the three primary weed species, all pathogens listed above were detected on weeds with the exception of A. cochlioides. The literature confirms the host status for these weeds for A. cochlioides, but was not observed over the course of this study. Polymyxa betae is a soilborne protozoan (formerly considered to be a primitive fungus) that transmits BNYVV to sugar beet plants. Although P. betae was detected from all three weeds species (Figure 1), the virus causing rhizomania (BNYVV) was never identified. The viral pathogen has been shown to infect various weed species in Europe, but is not thought to be important to the spread and inoculum increase for later sugar beet crops.
Disease symptoms observed on infected weeds were consistent with what would be expected on infected sugar beets or dry beans. In general, all root-infected weeds showed symptoms of wilting (Figure 2) and/or yellowing (Figure 3). Rhizoctonia produced small dark necrotic lesions (Figure 4) that coalesced to form larger rotted areas (Figure 5). Fusarium oxysporum induced vascular discoloration (Figure 6) while Pythium (most often P. aphanidermatum) tended to produce a tip rot of taproots (Figure 7) or small feeder roots (Figure 8). Cercospora beticola produced small circular-oval, grayish lesions with dark borders on lambsquarters (Figure 9). N. aberrans infections additionally produced galled roots on lambsquarters and Kochia that are identical to those on sugar beets (Figure 10). The Ramularia leaf spot (Figure 11) and downy mildew (Figure 12) diseases of lambsquarters also caused symptoms that would be very similar to infections in sugar beets.
Based on the severity of observed plant symptoms, some of the diseases on weeds, such as those caused by nematodes on lambsquarters or Pythium infections of pigweed roots, did not appear to severely damage weeds. However, it does illustrate the problem of potential pathogen carryover, and inoculum increase in fields in the absence of sugar beets or dry beans. It has long been observed that beans following beets in rotation often yield poorly. The reasons for this are not completely understood, but may be partially due to the fact that R. solani and Pythium spp. can cause disease in both crops. The damage by Ramularia sp. observed on lambsquarters does appear to be substantial, thus it could potentially be evaluated as a candidate for managing this weed through biological control as a mycoherbicide.
 |
 |
|
Figure 1. Overwintering honeycomb-like structures, called cystosori, of Polymyxa betae (vector of the sugar beet pathogen beet necrotic yellow vein virus) in small feeder roots of lambsquarters. |
Figure 2. Wilting symptoms in pigweed. |
 |
 |
|
Figure 3. Yellowing and wilting symptoms in pigweed. |
Figure 4. Individual necrotic lesions (left) from R. solani coalescing to form larger rotted areas in pigweed roots (right — top and bottom). |
 |
 |
|
Figure 5. Severe root rot (left) due to R. solani in Kochia (fireweed) roots compared to unaffected plant on right. |
Figure 6. Vascular discoloration of stems (top) and root (bottom) due to F. oxysporum in pigweed. |
 |
 |
|
Figure 7. Severe rot of pigweed taproot due to Pythium spp. |
Figure 8. Tip rot of feeder roots in pigweed due to Pythium spp. |
 |
 |
|
Figure 9. Cercospora leaf spot symptoms on lambsquarters (right) compared to healthy leaf on left. |
Figure 10. Galls on lambsquarters roots caused by the false root-knot nematode (N. aberrans). |
 |
 |
|
Figure 11. Ramularia leaf spot on lambsquarters. |
Figure 12. Downy mildew on lambsquarters. |
Summary and Conclusions
Rotating nonhost crops with dry edible beans and sugar beets has been shown to reduce the effects of several pathogens and their diseases on subsequent crops. Examples of this phenomenon include sugar beet cyst nematode, Aphanomyces root rot and Fusarium yellows in sugar beets and Fusarium yellows and root rot in dry beans. These particular diseases are specific to their respective crops. Therefore, by breaking the cropping cycle with nonhosts, the buildup of the pathogens causing disease should theoretically be reduced in these cases. Other pathogens such as Rhizoctonia and Pythium are less specific and more versatile in the plants they are capable of attacking; therefore, rotation would likely have little effect on reducing the severity of these diseases. However, in either case, the presence of infected weeds in fields could easily nullify the desired benefits of rotation for disease reduction by harboring or increasing certain pathogens that could subsequently attack new sugar beet and/or dry bean crops. The two newly identified diseases of lambsquarters are also apparently incapable of infecting sugar beets. However, infections on these plants within fields could still function as sentinels, suggesting that similar infections could occur on the beet crop plants due to favorable environmental conditions.
This publication has been peer reviewed.
Visit the University of Nebraska–Lincoln Extension Publications website for more publications.
Index: Plant Diseases
Field Crops
2007, Revised October 2013