G1562
Bacterial Wilt of Dry Beans in Western Nebraska
Bacterial wilt of dry beans has reappeared in Nebraska dry bean fields. This NebGuide addresses symptoms and identification, life cycle, and management of bacterial wilt in dry beans.
Robert M. Harveson, Extension Plant Pathologist
Carlos A. Urrea, Dry Bean Breeder
Howard F. Schwartz, Extension Plant Pathologist, Colorado State University
- Introduction
- Symptoms
- Pathogen Color Variants
- Infection and Survival
- Bacterial Wilt Importance and Management
- Management Techniques
- Conclusions
Introduction
Bacterial wilt of dry beans, caused by Curtobacterium flaccumfaciens pv. flaccumfaciens (Cff), has been a sporadic — but often serious — production problem in dry beans throughout the irrigated High Plains since first being reported in South Dakota in 1922. It was first observed in western Nebraska dry bean production fields in the early-mid 1950s, and continued to be an endemic, economically important problem throughout the 1960s and early 1970s. The disease then only periodically appeared in seed, but had little detectable effect on yields after the implementation of crop rotation and seed sanitation practices.
The pathogen was again identified in 2003 for the first time in this area in almost 25 years. Over the last seven to eight years, it has fully re-emerged in the Central High Plains (Nebraska, Colorado, and Wyoming) and has now been identified from more than 400 fields. Affected fields were planted with dry beans from multiple market classes and seed sources, including yellows, great northern, pintos, kidneys, cranberries, blacks, navies, pinks, and small reds. Disease incidence in these fields has varied from trace levels to >90 percent.
Symptoms
Field symptoms consist of leaf wilting (Figure 1) during periods of warm, dry weather or periods of moisture stress. This occurs because of the pathogen’s presence within the vascular system, which blocks normal water movement from roots into the foliage. Plants often recover during evening hours when temperatures are lower but wilt again during the heat of the day. Disease severity and plant mortality are often higher on young plants or those growing from infected seed. Seedlings are particularly susceptible, and if attacked when 2-3 inches tall, usually die (Figure 2). Symptoms on adult plants are less pronounced as the disease generally develops and progresses more slowly.
 |
 |
|
| Figure 1. Leaf wilting symptoms on dry beans due to bacterial wilt. | Figure 2. Death of a dry bean seedling due to bacterial wilt. |
Infected plants in Nebraska have additionally exhibited symptoms consisting of interveinal, necrotic lesions surrounded by bright yellow borders (Figure 3). These symptoms may be confused with those caused by common bacterial blight, Xanthomonas axonopodis pv. phaseoli, (synonym X. campestris pv. phaseoli) (Figure 4), but bacterial wilt lesions tend to be more wavy or irregular (Figure 5). Additionally, water soaking of leaves is not usually observed with wilt, as it is with common bacterial blight and halo blight (Pseudomonas syringae pv. phaseoli) infections. However, water soaking has commonly been observed on bacterial wilt infected yellow bean leaves in Colorado (Figure 6).
 |
 |
|
| Figure 3. Interveinal necrotic symptoms with irregular yellow halo, characteristic of wilt. | Figure 4. Marginal necrosis and yellowing symptoms associated with common bacterial blight. | |
 |
 |
|
| Figure 5. Necrotic and wavy yellow border symptoms associated with wilt. Note systemic and irregular nature to necrosis contrasted with common blight symptoms in Figure 4. | Figure 6. Water-soaking symptoms on yellow beans due to wilt. |
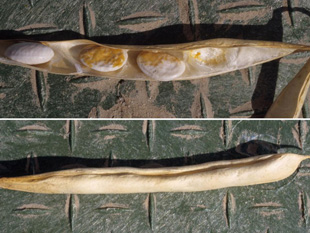
If plants survive to produce mature seed, the pathogen will often color or stain seeds (Figure 7), due to the systemic nature of this disease. Seeds may become infected even while pods appear to remain healthy (Figure 8). White-seeded cultivars are particularly prone to quality reductions due to the conspicuously-colored seed coats from systemic infections.
 |
 |
|
| Figure 7. Dry bean plant surviving to harvest but producing discolored seeds as a result of wilt infection. | Figure 8. One pod of wilt-infected dry bean plant showing discolored seeds (top) inside a pod with no external symptoms (bottom). Note that not all seeds are infected or discolored. |
Pathogen Color Variants
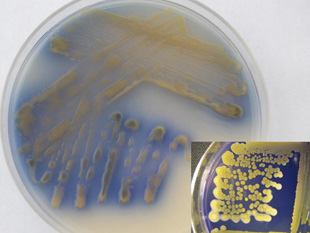
Colony growth and staining of seeds reported for original isolates of Cff were always yellow until orange- and purple-colored variants were found in western Nebraska. The purple variant maintains a yellow-colored colony in culture, but produces an extracellular, water-soluble bright purple pigment that diffuses into growth medium within two to three days (Figure 9) and also discolors seed. The purple variant, which is very rare, has only been reported once outside of the western Nebraska Panhandle — from cull bean seeds in Alberta, Canada, in 2006. The pigment produced by the purple variant is often unstable and inconsistently expressed, which may explain this variant’s lower reported incidence in nature.
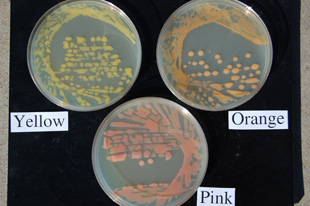
Since 2005, all three pathogen color variants have been isolated from infected dry bean seeds (Figure 10) and plants in western Nebraska fields during the season, with more than 90 percent of collected isolates during this time consisting of the yellow and orange variants (Figure 11). Following the 2007 growing season, a pink bacterial isolate (Figure 11) closely resembling the wilt pathogen was recovered on isolation media from orange-stained seeds (market class great northern) that originated from research plots affiliated with the University of Nebraska–Lincoln Panhandle Research and Extension Center (Scottsbluff Ag Lab) near Mitchell, Neb.
Infection and Survival
The pathogen is seedborne, and infected seeds represent the major source of inoculum and means for dispersal, both long and short distances. The pathogen can be transmitted both within and on the outside of seeds. It can overwinter on infected residue or weeds, but survival in soil by itself is poor. Due to a strong resistance to drying, the pathogen can remain viable up to 24 years in seed stored under optimum conditions in the laboratory.
Initial infection occurs when the pathogen enters the vascular system through either infected seed or through wounds on leaves or stems. Disease is not thought to develop via entry into numerous natural pores in leaves (stomata), unlike common bacterial and halo blights. However, the disease develops and spreads more rapidly, and becomes more severe following hailstorms (Figure 12), or when temperatures exceed 90°F. Wilting of plants is more pronounced during periods of moisture stress, and secondary spread occurs in a similar manner to that of common bacterial and halo blights.
 |
 |
|
| Figure 9. Young wilt pathogen culture exhibiting purple color variant. Note purple-blue pigments diffusing into media. Aged culture showing remnants of purple pigments in media (inset). Note also well-defined yellow color variant colonies. | Figure 10. Great northern dry bean seeds affected by pathogen color variants (clockwise from bottom) orange, purple, yellow, and uninfected. | |
 |
 |
|
| Figure 11. The pink wilt variant growing in culture compared to the typical yellow and orange variants on nutrient broth yeast extract medium (NBY). | Figure 12. Dry bean leaves damaged by hail. Note symptoms of wilt infection associated with the tattered leaves. |
|
The most important hosts for bacterial wilt are Phaseolus spp., especially P. vulgaris L. (common bean), but wilt isolates can survive and remain pathogenic in soil for at least two years. However, the primary mechanism for survival is in crop residues. A comprehensive survey over the last four years has further revealed the presence of bacterial wilt isolates occurring with other crops grown in rotation with dry beans, including soybeans, corn, wheat, sunflower, and alfalfa (Figure 13). These isolates were found in association with other bacterial diseases, suggesting survival in those crops’ residues.
Bacterial Wilt Importance and Management
Bacterial diseases of dry beans are very difficult to manage due to the lack of information on the effectiveness of chemical management options. Therefore, genetic resistance is generally considered to be the most effective means of disease management.
The resistant cultivar ‘Emerson’ was developed in the early 1970s by the University of Nebraska specifically for controlling bacterial wilt, which demonstrates the importance that this disease once held. ‘Emerson’ is still available today, but it is grown on a limited basis as a specialized variety for targeted markets in Europe. It cannot be produced on all fields where the disease has recently been identified. Thus, an emerging problem that needs addressing is utilizing newly developed resistant cultivars. New resistance studies have begun in Nebraska in which Phaseolus germplasm collections are being evaluated for resistance to Cff in the ongoing effort to produce new wilt-resistant cultivars adapted for dry bean production in this region.
Management Techniques
- Do not save seed from previously diseased fields for reuse.
- Plant certified seed of disease-tolerant cultivars where possible.
- Plant seed treated with streptomycin.
- Rotate beans with other crops for two to three years.
- Incorporate infected bean residues after harvest.
- Eliminate bean volunteers during the growing season.
- Stay out of bean fields when wet.
- Do not spread old bean straw from infested crops on new fields to be used for bean production.
- Avoid reusing irrigation water.
- Do not plant beans close to recently infected fields.
- Consider preventative sprays of a copper-based bactericide during mid-vegetative or early flowering periods, depending upon weather and potential pathogen involved.
Conclusions
Our studies to date suggest that the pathogen has re-emerged extensively throughout the dry bean production areas, due, in part, to recent changes in cultural practices. It is likely that it never disappeared completely but survived at low levels as a saprophyte on weed species or crop residues. Thus, it was not always noticed due to most fields being plowed (removing a source of survival) until recently. Also, the symptoms of wilt are additionally reminiscent of — and may be confused with — common bacterial blight.
Over the last 10-15 years, reduced tillage has become a widespread practice, as has the addition of higher numbers of center pivots in production fields. Both practices enhance conditions for the survival and spread of the pathogen within dry bean fields. This disease is also more severe under elevated levels of plant stress, illustrated throughout the mid-2000s during some abnormally hot and dry growing seasons. At this point, it is being hypothesized that the combination of environmental stress, changes in cultural practices, and unfamiliarity with the disease all contributed to the high visibility and increased incidence of this disease in the Central High Plains within the last eight years.
This publication has been peer reviewed.
Visit the University of Nebraska–Lincoln Extension Publications website for more publications.
Index: Plant Diseases
Dry Bean
2005, Revised June 2011
